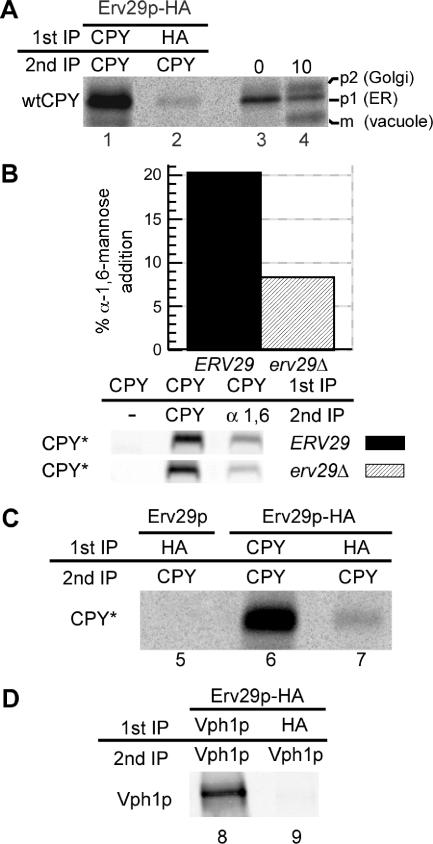
Figure 1.
ER exit of both wtCPY and misfolded CPY* is dependent on the ER export cargo receptor Erv29p. (A) erv29Δ cells (KHY271) expressing wtCPY and Erv29p-HA (pAC530) were grown at 30°C and radiolabeled with 35S methionine/cysteine for 6 min, and spheroplasts were treated with the cross-linking agent, DSP. Cell lysates were immunoprecipitated with anti-CPY (lane 1) or anti-HA antibodies (lane 2), the cross-linker was cleaved, and proteins reimmunoprecipitated with anti-CPY antibodies. Immunoprecipitated proteins were separated by SDS-PAGE and exposed to a phosphor screen. As markers of p1, p2, and mature wtCPY, KHY271 cells expressing Erv29 (pAC505) were grown at 30°C, radiolabeled for 5 min, and chased with unlabeled methionine/cysteine. Samples were removed at indicated times, and cell extracts were prepared, and wtCPY was immunoprecipitated with anti-CPY antibodies (lanes 3 and 4). Proteins were resolved by SDS-PAGE. (B) ERV29 (KHY171) and erv29Δ cells (KHY279) expressing CPY* (pAC519) were radiolabeled for 20 min and chased for 5 min, and CPY* was immunoprecipitated. The samples were solubilized, reimmunoprecipitated with either anti-CPY or anti-α-1,6-mannose antibodies, and treated with Endo H followed by SDS-PAGE and quantification. Graphic representation of the α-1,6-mannose modification of CPY* in ERV29 and erv29Δ cells is shown. (C) erv29Δ (KHY270) cells expressing Erv29p (pAC505, lane 5) or Erv29p-HA (pAC530, lane 6 and 7) were radiolabeled for 10 min before cross-linking and immunoprecipitation with anti-HA or anti-CPY antibodies and then reimmunoprecipitated with anti-CPY antibodies. Samples were treated with Endo H before SDS-PAGE. (D) erv29Δ (KHY270) cells expressing Vph1p (pMM322) and Erv29p-HA (pAC530) were radiolabeled for 6 min before cross-linking and immunoprecipitation with anti-HA or anti-Vph1 antibodies and then reimmunoprecipitated with anti-Vph1 antibodies.