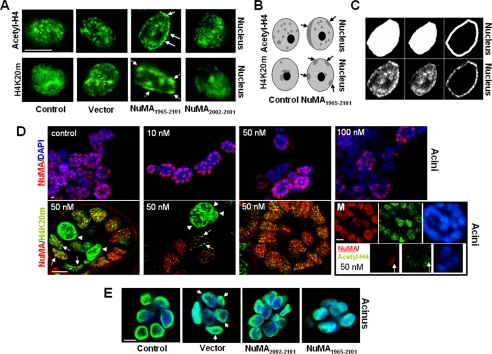
Figure 7.
Altered NuMA induces changes in higher order chromatin organization. (A) NuMA1965–2101-S1, NuMA2002–2101-S1, insertless vector-S1, and (nontransfected) control-S1 cells were cultured for 10 d in 3D to induce acinar morphogenesis. Shown is immunostaining for chromatin markers acetyl-H4 and H4K20m. Arrows point to alterations (i.e., peripheral accumulation) in the staining of chromatin markers. (B) Drawings representing the alterations in the higher order organization of acetyl-H4 and H4K20m commonly observed in NuMA1965–2101- S1 cells. Light gray indicates the diffuse staining for these proteins and dark gray indicates the concentration of acetyl-H4 and H4K20m in specific areas of the cell nucleus. Arrows indicate acetyl-H4 and H4K20m domains that concentrate at the nuclear periphery upon expression of the C terminus of NuMA. (C) Images depicting the procedure followed using MetaMorph (method 2) to quantify alterations in chromatin organization upon alteration of NuMA. Immunostaining for acetyl-H4 (bottom) and masks created using MetaMorph (top), corresponding from left to right to the whole nucleus, interior of the nucleus, and peripheral portion of the nucleus are shown for one nucleus (for a full explanation of the procedure, see Materials and Methods). (D) Twenty hours posttransfection of S1 monolayer cultures with siRNA. S1 cells were placed in 3D culture for 8 d to induce acinar morphogenesis. Top, immunostaining for NuMA (red) and DNA counterstaining (blue) in S1 acini untreated (control), and S1 acini incubated with increasing concentrations of NuMA siRNA (10, 50, and 100 nM). The number of nuclei only stained in blue (i.e., no NuMA staining) increases as cells are treated with higher concentrations of NuMA siRNA. Bottom, dual staining for NuMA (red) and H4K20m (green) in cells transfected with 50 nM NuMA siRNA. Three distinctive types of multicellular structures are shown from left to right: One acinus in which NuMA is expressed in some cells and absent in two cells (first image; arrowheads point to cells in which NuMA is absent); one cellular structure that did not develop into an acinus, from which NuMA is absent (top of the second image; arrowheads); and an acinus in which NuMA was not silenced (third image). Arrowheads indicate the strong peripheral concentration of H4K20m in the nucleus of cells that lack NuMA staining (one arrowhead per cell). Arrows indicate the presence of H4K20m domains at the nuclear periphery in cells that have a weak staining for NuMA. The montage (M) shows two multicellular structures taken from the same recorded image of a cell population transfected with 50 nM NuMA siRNA and stained for NuMA (red), acetyl-H4 (green), and DNA (DAPI, blue). The large multicellular structure (top three images) has strong staining for both NuMA and acetyl-H4, whereas the small multicellular structure (bottom three images) in which NuMA is predominantly absent shows very weak acetyl-H4 staining. Arrows point to a nucleus in which a remnant of NuMA expression coincides with a stronger staining for acetyl-H4. (E) NuMA1965–2101-S1, NuMA2002–2101-S1, insertless vector-S1 cells, and (nontransfected) control-S1 cells were cultured for 10 d in 3D to induce acinar morphogenesis. Shown is the distribution of dUTP (green) upon in situ nick translation. Nuclei are counterstained with DAPI (blue). Bar, 5 μm.