Figure 1.
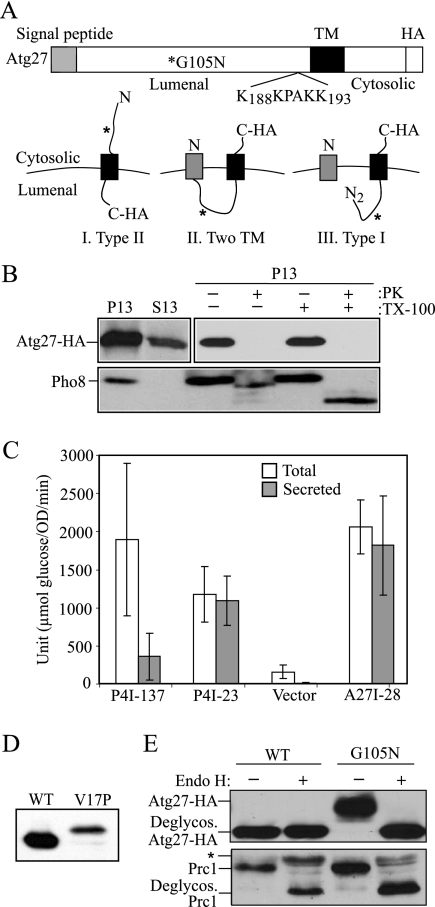
Atg27 is a type I transmembrane protein. (A) Schematic drawing of full-length Atg27. The full-length Atg27 protein contains 271 amino acids. Analysis of the primary amino acid sequence using the SignalP program indicates that Atg27 has a signal sequence (residues 1-19) and a transmembrane (TM) region (residues 199-221) according to TMHMM-prediction of helices in proteins (http://www.cbs.dtu.dk/services/TMHMM-2.0/), in a type I membrane topology. The putative PtdIns(3)phosphate-binding site (residues 188–193, KKPAKK) from the previous report (Wurmser and Emr, 2002) is indicated. Three possible membrane topologies for Atg27 are shown: I, Type II transmembrane orientation; II, two transmembrane domains; and III, Type I transmembrane protein. The asterisk marks the mutation introduced to replace glycine at position 105 with asparagine, creating a glycosylation site. (B) The C terminus of Atg27 is exposed to the cytosol. Atg27-HA (WLY1) and pep4Δ (TVY1) cells were converted into spheroplasts and then osmotically lysed. The cell lysates were centrifuged at 13,000 × g for 10 min to generate the pellet (P13) and supernatant (S13) fractions. The P13 fractions then were resuspended and subjected to treatment with 1% Triton X-100, proteinase K, both or neither on ice for 30 min. The lysates were then TCA-precipitated and analyzed by SDS-PAGE and Western blot. (C) The N-terminal 28 amino acids of Atg27 are able to direct invertase secretion. Cells expressing invertase fusion proteins (P4I-137, P4I-23, empty vector [pSEYC306], or A27I-28) were grown to early log phase. The cells were collected and subjected to an invertase activity assay as described in Materials and Methods. (D) The signal sequence of Atg27 is cleaved. Cells expressing Atg27-HA (WT) or Atg27V17P-HA (V17P) from the pAtg27–3xHA(416) or pAtg27V17P-3xHA(416) plasmids were grown to early log phase. The protein extracts were analyzed by Western blot and probed with monoclonal anti-HA antibody. (E) The lumenal region of Atg27 translocates into the ER. Cells expressing Atg27-HA (WT) and Atg27G105N-HA (G105N) were grown to early log phase. The cell lysates were subjected to endoglycosidase H treatment as described in Materials and Methods. After resolution by SDS-PAGE, the samples were analyzed by Western blot and probed with antibodies against Prc1 and HA, separately. The positions of glycosylated and deglycosylated forms of both proteins are indicated. The asterisk indicates cross-reacting bands.