Figure 3. The localization of the calmodulin binding site on arrestin2 using site-directed spin labeling EPR spectroscopy.
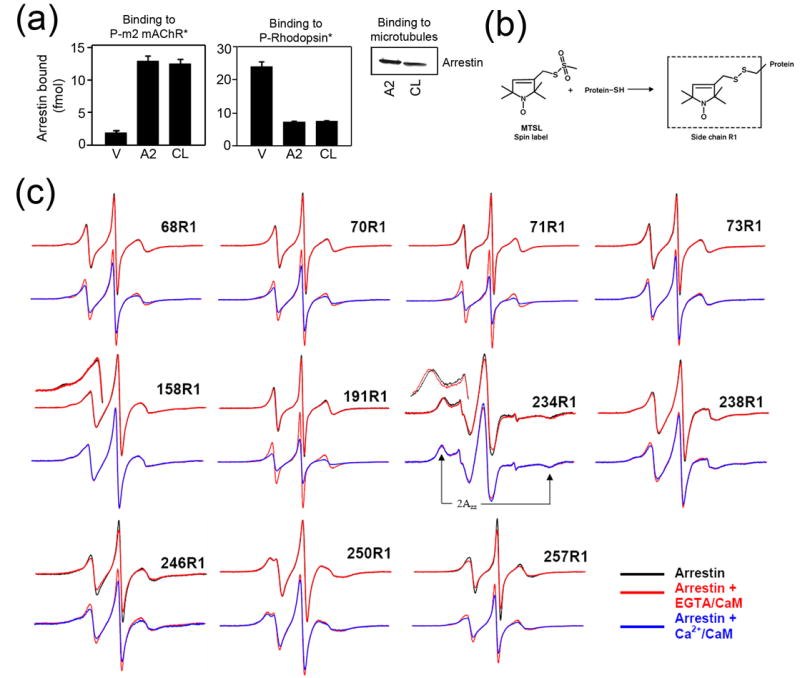
(a) Binding of visual (V), arrestin2 (A2), and cysless arrestin2 (CL) to the phosphorylated carbachol-activated m2 muscarinic receptor (P-m2 mAChR*) (left panel), light-activated phosphorylated rhodopsin (P-Rhodopsin*) (center panel), and microtubules (right panel) was performed as described in the Methods. Functionally cysless arrestin2 was identical to wild type arrestin2 in all cases. (b) The R1 side chain generated by reacting the arrestin cysteine mutants with the methanethiosulfonate (MTSL) nitroxide spin label reagent. (c) For each spin-labeled arrestin, normalized spectra in the absence (black) or presence of CaM + 1mM EGTA (red) are compared in the top row, and spectra in the presence of CaM + 1mM EGTA (red) and CaM + 0.1mM Ca2+ (blue) are compared in the bottom row. Portions of the overlaid spectra for 158R1 and 234R1 are magnified to better illustrate the spectral changes and the location of the hyperfine splitting (2Azz'). The spectrum of each arrestin mutant alone in solution or with 1mM EGTA or 0.1mM Ca2+ in the absence of calmodulin (black) were identical (spectra not shown). Only the spectra showing significant changes in the presence of calmodulin are presented.
