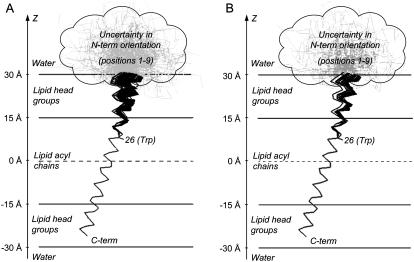
FIGURE 4.
(A) Resulting 100 structures obtained from global analysis of experimental FRET data of AEDANS-labeled M13 coat protein mutants in DOPC/DOPG vesicles. The structures are presented in terms of Cα positions that are projected on the plane formed by the OZ axis and the direction of tilt of the transmembrane domain. The protein domain from amino acid residue 1–9 cannot be described by a rigid α-helix and is schematically presented as a “cloud” containing several gray “unstructured” conformations. (B) Final set of 52 structures obtained after fitting of experimental data and filtering using Stokes shift information. The resulting tilt angle of the N-terminal domain ϕ = 5.0 ± 4.7°.