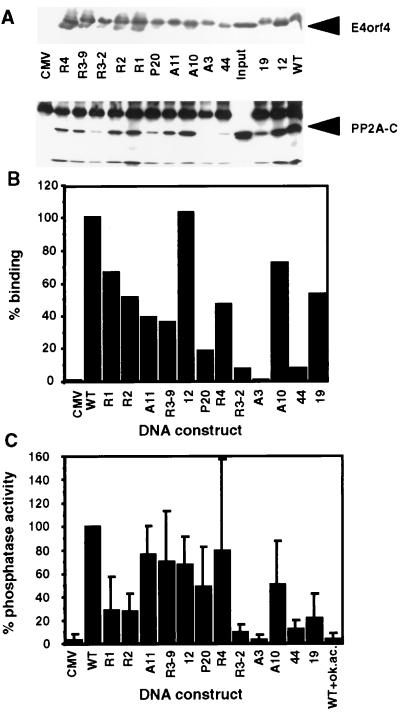
Figure 2.
Binding of PP2A to E4orf4 mutants. (A) Mutant E4orf4 proteins were immunoprecipitated from 293T cells transfected with the mutant DNA constructs or the empty CMV vector (CMV). The immune complexes were detected on a Western blot by antibodies specific for E4orf4 and the PP2A-C subunit. (B) The intensity of PP2A binding was determined by densitometry of the Western blot shown in A. The binding of PP2A to the wt E4orf4 protein was defined as 100%. Mutants are arranged according to the location of the mutations, from the amino to the carboxyl terminus. (C) E4orf4 mutant proteins were immunoprecipitated, and phosphatase activities associated with the immune complexes were determined. Phosphatase activity associated with the wt E4orf4 protein was defined as 100%. The average of four experiments is shown. Background levels were determined either in the absence of E4orf4 (CMV) or in a phosphatase reaction carried out in the presence of 4 nM okadaic acid (WT+ok. ac.).