Figure 5.
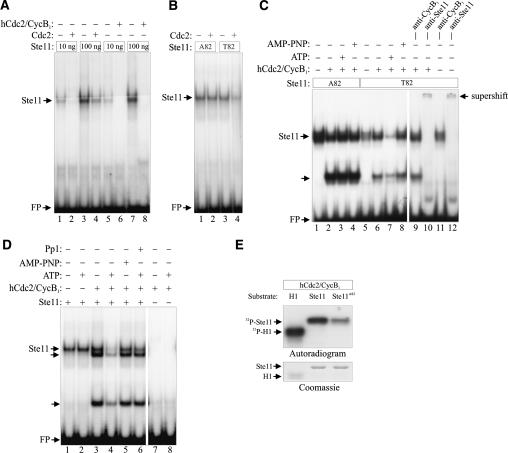
Cdc2 inhibits the DNA-binding activity of bacterially produced Ste11. (A) Purified GST-Ste11 (10–100 ng) was either directly assayed by EMSA for DNA-binding activity or was first incubated with either fission yeast Cdc2 immune complexes or with 2.5 ng (50 U) of recombinant human Cdc2–Cyclin B1 complex (New England Biolabs) in the presence of ATP. (B) Ten nanograms of GST-Ste11 and GST-Ste11T82A were either directly assayed by EMSA for DNA-binding activity or were first incubated with ATP and Cdc2 immunoprecipitated from fission yeast cells. (C) Twenty-five nanograms of GST-Ste11 and GST-Ste11T82A were either directly assayed by EMSA for DNA-binding activity or were first incubated with 0.5 ng of human Cdc2–Cyclin B1 complex in the presence or the absence of ATP or AMP-PNP. In addition, lanes 9 + 11 and lanes 10 + 12 contained anti-Cyclin B1 and anti-Ste11 antibodies, respectively. (D) In a similar assay, the effect of adding Protein Phosphatase 1 (Pp1) to Cdc2–Cyclin B1-phosphorylated GST-Ste11 was examined (cf. lanes 4 and 6). In addition the DNA-binding activity of Cdc2–Cyclin B1 was assayed in the absence of GST-Ste11 (lanes 7 + 8). (E) In vitro kinase assay. Protein kinase activities of recombinant human Cdc2–Cyclin B1 were measured using histone H1 (H1), wild-type Ste111–113 (Ste11), and Ste11T82A1–113 (Ste11T82A) as substrates. Kinase assays were carried out for 15 min at 30°C, and the samples were separated by SDS-PAGE, followed by autoradiography and Coomassie staining.