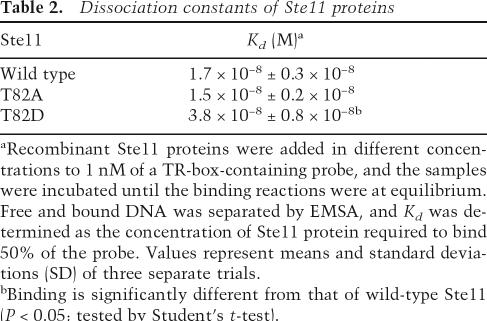
Table 2.
Dissociation constants of Ste11 proteins
aRecombinant Ste11 proteins were added in different concentrations to 1 nM of a TR-box-containing probe, and the samples were incubated until the binding reactions were at equilibrium. Free and bound DNA was separated by EMSA, and Kd was determined as the concentration of Ste11 protein required to bind 50% of the probe. Values represent means and standard deviations (SD) of three separate trials.
bBinding is significantly different from that of wild-type Ste11 (P < 0.05; tested by Student’s t-test).