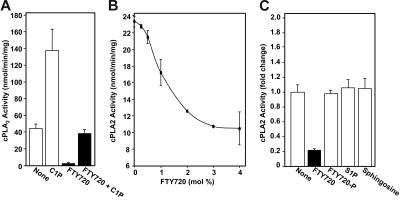
Figure 7.
FTY720 reduces the enzymatic activity of recombinant cPLA2α. (A) Recombinant cPLA2α (0.5 μg) activity was measured in the absence or presence of 4 mol% FTY720 or C1P, or 2 mol% of each in a mixed micelle assay. Data are means ± SD of duplicate determinations expressed as nanomole AA released per minute per milligram. (B) Stoichiometry of cPLA2 inhibition by FTY720. Recombinant cPLA2α (0.5 μg) activity was measured in the absence or presence of increasing mol% FTY720 (100 × [FTY720]/[Triton X-100 + PC + FTY720]). The PC concentration was fixed at 15 mol% (100 × [PC]/[Triton X-100 + PC + FTY720]). Data are means ± SD of duplicate determinations expressed as nanomole AA produced per minute per milligram. Nonlinear regression analysis was performed (R2 = 0.999). The IC50 value for FTY720 inhibition of cPLA2α activity was 1.1 ± 0.03 mol%, and the Hill coefficient was 2.3 ± 0.1. (C) Lack of effect of sphingoid bases on cPLA2α activity. Recombinant cPLA2 (0.5 μg) activity was determined in the absence or presence of 4 mol% FTY720, FTY720-P, sphingosine, or S1P. Data are expressed as fold change in cPLA2α activity compared with control activity (none) and are means ± SD of triplicate determinations. Similar results were obtained in 3 independent experiments.