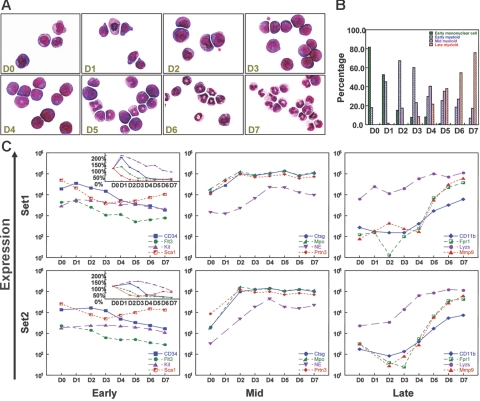
Figure 1.
G-CSF–driven myeloid differentiation from day 0 to day 7. (A) Morphology of G-CSF–treated cells from day 0 to day 7. Cytospins were made with cells harvested on days 0 to 7, and differentials were performed after May-Grunwald-Giemsa staining. (Sigma, St. Louis, MO). Cells were visualized using a Nikon Microphot-SA microscope (Nikon, Tokyo, Japan) with a 100×/1.40 oil objective. Images were captured using a Colorview II camera (Soft Imaging System, Lakewood, CO), and images were produced with analySIS software (Soft Imaging System). The images were not edited. (B) Differential counts for early mononuclear cells, early myeloid cells (promyelocytes), mid myeloid cells (myelocytes and metamyelocytes), and late myeloid cells (bands and neutrophils) from day 0 to day 7. Two hundred cell differentials were performed for each day. (C) Changes in the expression of known early, mid, and late myeloid genes during G-CSF–driven myeloid differentiation in 2 independent gene expression analyses (set 1 and set 2). “Early” genes include CD34, fms-related tyrosine kinase 3 (Flt3), Kit, and Sca1; “mid” genes include cathepsin G (Ctsg), myeloperoxidase (Mpo), neutrophil elastase (Ela2), and proteinase 3 (Prtn3); “late” genes include CD11b (Itgam), formyl peptide receptor 1 (Fpr1), lysozyme (Lyzs), and Mmp9. Signal intensity values (normalized to whole array target intensity of 1500 for all probe sets) are displayed on the y-axis. The inset in the “early” panel displays expression values on a linear scale instead of a log scale.