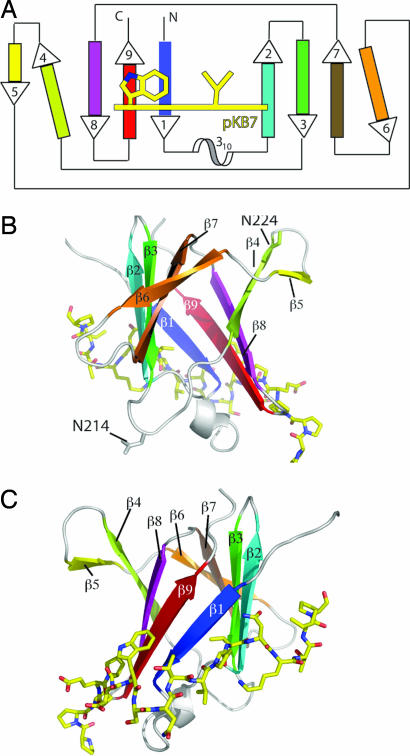
Fig. 1.
Overview of TraOCT/TraN complex structure. (A) Schematic topology diagram of TraOCT with β-strands color-ramped as in SI Fig. 7C. The 310 helix is colored white. TraN is represented as a yellow line crossing strands β1, β2, and β9 of TraOCT. Residues Trp-27 and Val-33, two major contact points with TraOCT, are shown. Strands β4 and β6 are shown at an angle to their adjacent strands to reflect their weaker H-bonding interactions. (B) Rotation of the model through 180° about the vertical axis with respect to C to show strands β4, β5, and β6 on the opposite face to the TraN-binding site. Residues N214 and 224 (N216 and N226 in A. tumefaciens) VirB9 are shown in ball-and-stick representation. (C) Stereo diagram of structure showing TraOCT in cartoon representation with loops, 310 helix, and β-strands colored as in A. TraN is shown as a stick model with carbon atoms colored yellow, nitrogen atoms colored blue, and oxygen atoms colored red. The same representations and color scheme is used in B and C. PyMOL was used for all structure figures (www.pymol.org).