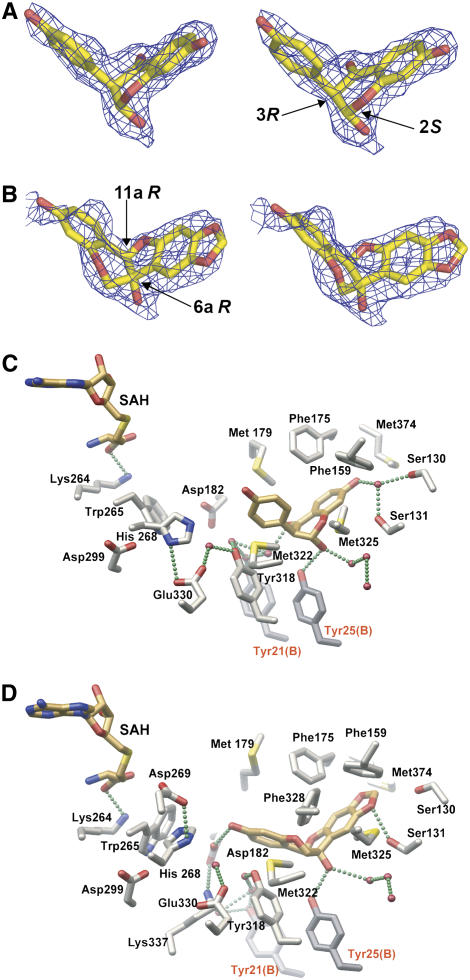
Figure 8.
Close-Up Views of the Complexes of HI4′OMT with 2,7,4′-Trihydroxyisoflavanone and 6aR,11aR-6a-Hydroxymaackiain.
(A) Stereo view of the electron density associated with the 2S,3R-stereoisomer of 2,7,4′-trihydroxyisoflavanone observed crystallographically in the HI4′OMT complex. The SIGMAA-weighted composite-omit electron density map was contoured at 1.2 σ. While the axial positions of the hydroxy-phenyl and hydroxyl moieties clearly represent higher-energy conformations, HI4′OMT presumably uses binding energy to selectively sequester the 2,7,4′-trihydroxyisoflavanone substrate.
(B) Stereo view of the electron density associated with 6aR,11aR-6a-hydroxymaackiain observed crystallographically in the HI4′OMT complex. The SIGMAA-weighted composite-omit electron density map was contoured at 0.6 σ due to lower occupancy of this bound pterocarpan. Given the lower occupancy of 6aR,11aR-6a-hydroxymaackiain obtained by soaking into HI4′OMT crystals with SAH already bound (no opportunity for methylation to occur), there is some deviation of the refined coordinates for 6aR,11aR-6a-hydroxymaackiain from the electron density.
(C) Close-up view of the HI4′OMT substrate/product binding site with SAH and 2S,3R-2,7,4′-trihydroxyisoflavanone shown. Bonds are color-coded by atom type, with SAH and isoflavanone carbon atoms in gold and protein carbon atoms in gray. Oxygen atoms are red, nitrogen atoms are blue, and sulfur atoms are yellow. Two amino acid residues from the dyad-related monomer B are labeled with red lettering. The putative hydrogen bonds are depicted as green spheres.
(D) Close-up view as in (C) illustrating the conformation and location of bound 6aR,11aR-6a-hydroxymaackiain. For clarity, the active site residues Asp-269, Phe-328, and Lys-337 that only participate in a hydrogen-bonding network in the HI4′OMT-SAH-6a-hydroxymaackiain complex shown in (D) were omitted in (C).