Table 2.
Thermodynamic Parameters of Association for At-OASS and C10 Peptide
| Kd (nM) | 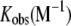 |
ΔH | −TΔS | ΔG | |
|---|---|---|---|---|---|
| Wild type | 5.6 ± 2.0 (37 ± 12) | 1.8 ± 0.8 × 108 (2.7 ± 0.9 × 107) | −9.7 ± 0.3 (−1.3 ± 0.4) | −1.6 (−8.8) | −11.3 ± 0.3 (−10.1 ± 0.2) |
| T74S | 100 ± 11 | 9.4 ± 0.1 × 106 | −5.8 ± 0.17 | −3.6 | −9.5 ± 0.08 |
| S75A | 40 ± 3 | 2.5 ± 0.2 × 107 | −9.2 ± 0.12 | −0.8 | −10.0 ± 0.05 |
| S75T | 1471 ± 50 | 6.8 ± 0.5 × 105 | −5.9 ± 0.3 | −2.0 | −7.8 ± 0.08 |
| Q147A | 56 ± 13 | 1.8 ± 0.3 × 107 | −8.8 ± 0.3 | −0.9 | −9.9 ± 0.08 |
Values of ΔH, TΔS, and ΔG are in kcal mol−1. Wild-type data were fit to the equation describing a two-site binding, with the parameters for the second binding site shown in parentheses. Mutant data were fit to the equation describing a single-site binding model.
