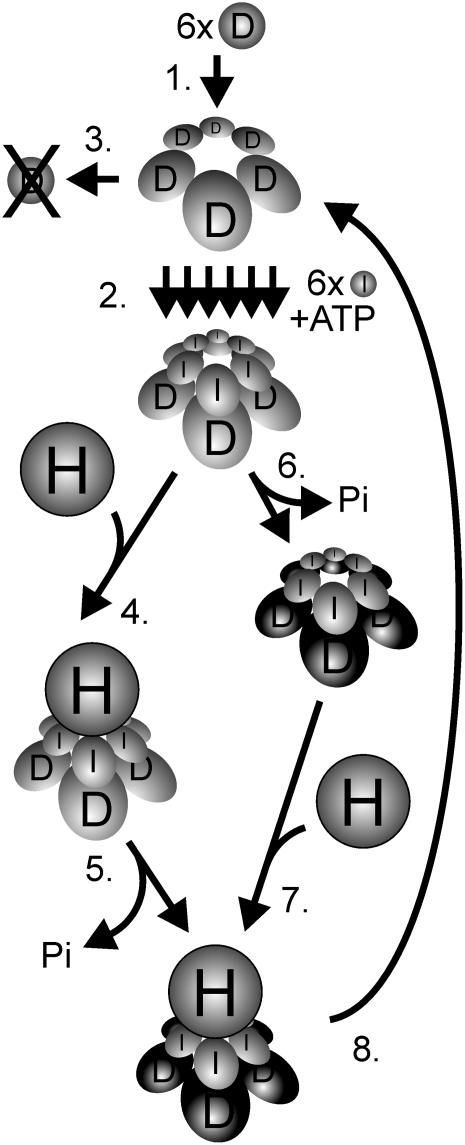
Figure 7.
Model of the Catalytic Cycle of Mg-Chelatase with Focus on the D Subunit.
1, Six D subunits form a hexameric ring structure in an ATP-independent process. 2, In a Mg2+- and ATP-dependant process, six I subunits are stepwise assembled into a hexameric ring using the D hexameric structure as a platform. The two-tiered hexameric ring also forms in the presence of ADP, but further steps are abolished. 3, In the absence of functional I subunits, the D subunits are degraded. 4, Timing of ATP hydrolysis in relation to the addition of the H subunit is not established. From structural data, it is suggested that the ATP hydrolysis is stalled in the ID complex and, 5, triggered upon binding to the H subunit (Fodje et al., 2001). The H subunit holds the protoporphyrin IX and most likely also the Mg2+ substrate and has therefore been considered as the catalytic subunit. 6, From studies with ATPase-deficient I mutants, it seems that the formation of an ID complex is not enough to rescue the D subunit from degradation (Lake et al., 2004). Instead, a concerted ATPase-dependent action of the I proteins is likely to perform a structural change of the D proteins. After the conformational change, the D proteins are safe from degradation and, 7, bind to the H subunit. 8, After the formation of Mg-protoporphyrin, the complex disassembles except for the hexameric D ring, which is ready to function as a platform in a new catalytic cycle. The concerted behavior of the D hexamer could be expressed in step 6 if the cooperative activity of the I subunits required a concerted structural change of its D hexameric substrate. Alternatively, the concerted activity of the D hexamer is required in the catalytic interaction with the H subunit in steps 5 or 7. At present, it is unknown whether the H subunit docks to the I or D side of the complex, although docking to the I subunits is shown in the figure.