Figure 6.
Movement of Full-Length Transcripts of St BEL5 across a Graft Union and Its Effect on Tuber Yield.
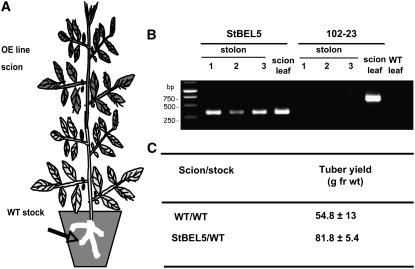
Soil-grown wild-type stock plants were grafted with scions from an St BEL5 overexpression line (5FL1-3) in the greenhouse. Both stock and scion material were grown initially under LDs. Grafts were sealed with plastic soda straw sections. Plants were placed in plastic bags, and graft unions were allowed to form. After 4 weeks of LDs in the greenhouse, grafted plants were transferred to a growth chamber and acclimated under LD conditions for 1 week before transfer to SD conditions. Leaf and stolon tip samples (arrow in [A]) were harvested after 12 d of SD conditions and the RNA extracted. RT-PCR with gene-specific primers was performed for both a negative control (a potato MADS box gene, 102-23) and test samples (St BEL5-FL). Grafts made from an overexpression line for an antisense sequence of a potato MADS box gene (line 102-23) were used as a nonmobile control. RNA from scion leaf samples was used as a positive control (scion leaf). Wild-type RNA from stolon tips, 0.5 cm in length, was sampled for both heterografts and used in the RT-PCR reactions. PCR was performed twice off template made from RNA and reverse transcriptase. Two different gene-specific primers were used with a nonplant DNA tag specific for the transgenic RNA to discriminate from the native RNA. Three plants were assayed for both heterografts and are designated 1, 2, and 3. RNA from leaves of a wild-type/wild-type autograft was used as a negative PCR control (WT leaf lane). Similar negative results were obtained with RNA from autograft stolons. For tuber yields, plants were harvested after 28 d, and the mean of three plants was calculated for wild-type and St BEL5 grafted plants. Wild-type scions grafted onto wild-type stocks were used as the yield controls. Identity of PCR products was confirmed by blot hybridization with a gene-specific probe.