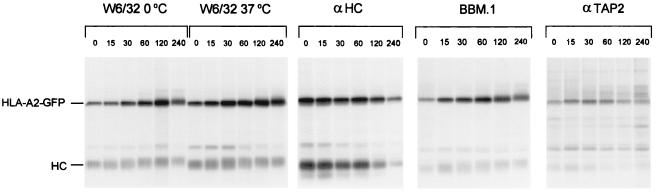
Figure 2.
GFP tagging does not affect assembly and transport of HLA-A2. To determine whether cytoplasmic tagging of the HLA-A2 heavy chain with GFP affects its maturation and transport, a pulse–chase experiment was performed. Mel JuSo stably transfected with HLA-A2-GFP was biosynthetically labeled for 15 min followed by culture for the times indicated. Cells were lysed, and the lysate was split into three equal aliquots. Aliquots 1 and 2 were incubated for 1 h at 0°C and 37°C, respectively and MHC class I complexes were immunoprecipitated with the conformation-specific antibody W6/32. Subsequently, aliquots 1 and 3 were immunoprecipitated with anti-heavy chain and anti-human β2m antibodies, respectively. Aliquot 3 was kept at 4°C and immunoprecipitated with anti-TAP2 serum. All samples were analyzed by 10% SDS/PAGE. The position of endogenous class I (HC) and GFP-tagged HLA-A2 (HLA-A2-GFP) are indicated. HLA-A2-GFP heavy chains associate with β2m (lanes W6/32 0°C, αHC, and BBM.1) to form complexes with stably bound peptides (lanes W6/32 37°C). In addition, HLA-A2-GFP-containing MHC class I complexes still are able to bind transiently to the TAP transporter (lanes αTAP2). HLA-A2-GFP containing complexes behave like endogenous complexes with respect to maturation, assembly, and TAP dissociation.