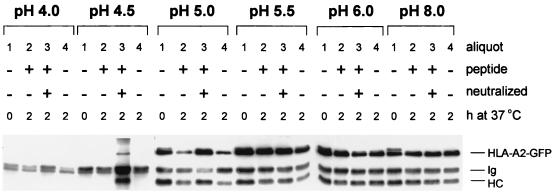
Figure 4.
pH-dependent peptide release and MHC class I dissociation. Mel JuSo HLA-A2-GFP cells were stimulated for 72 h with 200 units/ml of IFN-γ lysed in 1% NP-40 lysis buffer at pH 4.0, 4.5, 5.0, 5.5, 6.0, or 8.0, and each lysate was split into four equal aliquots. No peptide was added to aliquots 1 and 4. HPV16 E7-derived HLA-A2-binding peptide was added to aliquot 2, and HPV16 E7-derived HLA-A2-binding peptide + Tris⋅HCl, pH 7.4 was added to aliquot 3. All aliquots were incubated for 1 h on ice, and subsequently aliquots 2, 3, and 4 were incubated for 2 h at 37°C, while aliquot 1 remained on ice. Then 1 ml of NP-40 lysis buffer, pH 7.4 was added to each aliquot, and MHC class I complexes were immunoprecipitated with the conformation-specific antibody W6/32 without prior preclearing. The samples were analyzed by Western blotting after separation by 10% SDS/PAGE using anti-heavy chain antibodies. The position of endogenous class I (HC), GFP-tagged HLA-A2 (HLA-A2-GFP), and antibody heavy chain (Ig) is indicated. At a pH lower than 5.0 MHC class I complexes dissociate completely. At pH 5.0 peptide is dissociating from MHC class I complexes as is visualized by the decrease in temperature stable complexes that can be retrieved by W6/32.