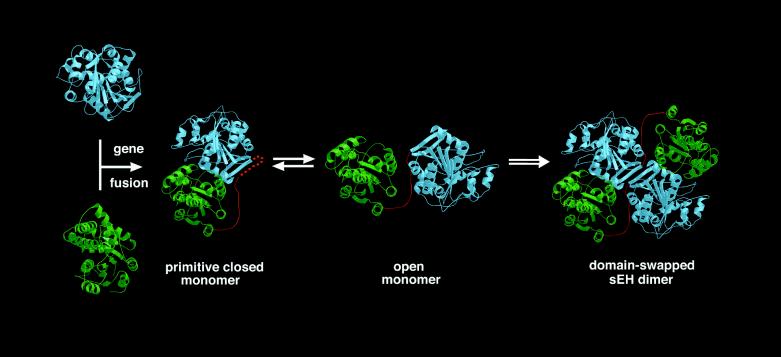
Figure 5.
Proposed structure-based evolutionary pathway of xenobiotic catabolism. Haloalkane dehalogenase (blue) and haloacid dehalogenase (green) ancestors underwent an early gene fusion event to yield a primitive monomeric protein adopting a postulated closed conformation. Subsequent equilibration with an open conformation and dimerization through domain-swapping is facilitated by a flexible linker (i.e., hinge loop; red); stabilization of the modern-day, domain-swapped sEH dimer is achieved through subsequent shortening and/or amino acid substitutions that rigidify the 16-residue linker, which in sEH contains 5 proline residues. This figure was prepared with bobscript and Raster3D (47–49).