Abstract
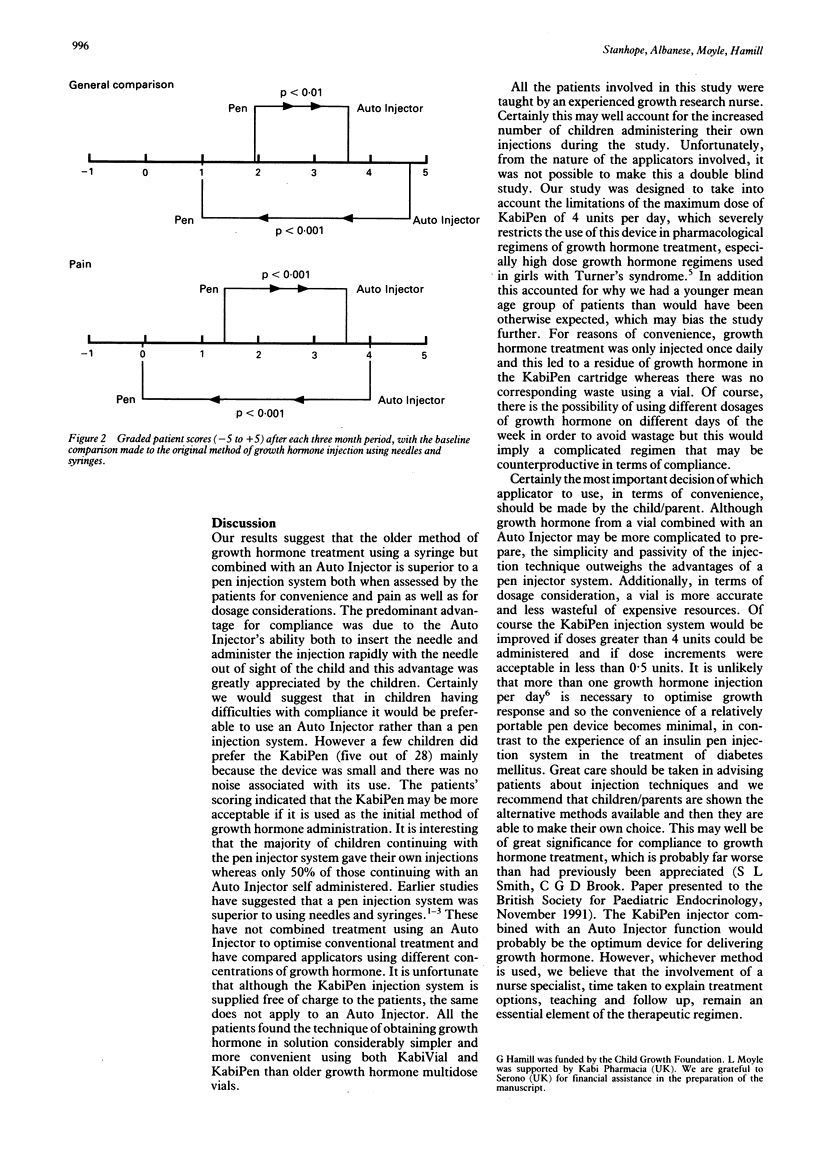
The use of optimum conventional growth hormone administration, using a growth hormone vial combined with an Auto Injector, was compared with a pen injection system using a cartridge of growth hormone. In both methods of administration the concentration of growth hormone was 16 IU/ml. Thirty patients (22 boys, eight girls) who had all previously been treated with growth hormone (4 IU/ml) administered using needles and syringes (without an Auto Injector) were randomised into receiving one of either treatment for three months and then crossed over for a further three months. Fourteen patients (10 boys, four girls) initially received KabiVial 16 IU/ml combined with an Auto Injector while 16 patients (12 boys, four girls) were treated with KabiPen 16 IU/ml. Mean age in both groups was 9.6 years. The majority of patients in both groups were treated with a regimen of either 15 or 20 units/m2/week as a daily subcutaneous injection. Of the 30 patients who started in this trial, two who commenced using an Auto Injector refused to change to a pen system and were excluded from further analysis. When scored on a scale of -5 to +5 general convenience when changing from an Auto Injector to the KabiPen decreased from +4.7 to +1.0. When assessed for pain, the Auto Injector group scored +4.7, which decreased to -0.2 (more painful) for the pen. At the end of the trial 23 patients (82%) chose to continue with the KabiVial/Auto Injector combination as they found this less painful and the child did not see the needle or need to insert the needle manually. Five patients (18%) continued with the KabiPen as they considered the device smaller and easier to use. The accuracy of dosing using KabiVial was 100% compared with the range of 88% to 111% using KabiPen as the latter was available only in 0.5 unit increments. No growth hormone was wasted using KabiVial, although a mean of 0.6 units was wasted with every 16 IU cartridge in the KabiPen system. It is concluded that patients should be able to contribute to the choice of growth hormone delivery systems and that newer methods need careful assessment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aicardi J. Clinical approach to the management of intractable epilepsy. Dev Med Child Neurol. 1988 Aug;30(4):429–440. doi: 10.1111/j.1469-8749.1988.tb04769.x. [DOI] [PubMed] [Google Scholar]
- Albertsson-Wikland K. Simplified growth hormone therapy--first clinical experience with the KabiPen. Acta Paediatr Scand Suppl. 1988;343:103–106. doi: 10.1111/j.1651-2227.1988.tb10809.x. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Cutfield W. S. Evaluation of a pen injector system for growth hormone treatment. Arch Dis Child. 1991 Jun;66(6):686–688. doi: 10.1136/adc.66.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill G., Stanhope R. Evaluation of a pen injector system for growth hormone treatment. Arch Dis Child. 1991 Dec;66(12):1466–1466. doi: 10.1136/adc.66.12.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarsh P. C., Stanhope R., Preece M. A., Brook C. G. Frequency of administration of growth hormone--an important factor in determining growth response to exogenous growth hormone. Horm Res. 1990;33 (Suppl 4):83–89. doi: 10.1159/000181590. [DOI] [PubMed] [Google Scholar]
- Jørgensen J. O., Møoller J., Jensen F. S., Jøorgensen J. T., Christiansen J. S. Growth hormone administration by means of an injection pen. Pharmacol Toxicol. 1989 Aug;65(2):96–99. doi: 10.1111/j.1600-0773.1989.tb01135.x. [DOI] [PubMed] [Google Scholar]
- Polkey C. E. Surgery for epilepsy. Arch Dis Child. 1989 Feb;64(2):185–187. doi: 10.1136/adc.64.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. J., Hindmarsh P. C., Brook C. G. Contribution of dose and frequency of administration to the therapeutic effect of growth hormone. Arch Dis Child. 1988 May;63(5):491–494. doi: 10.1136/adc.63.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]



