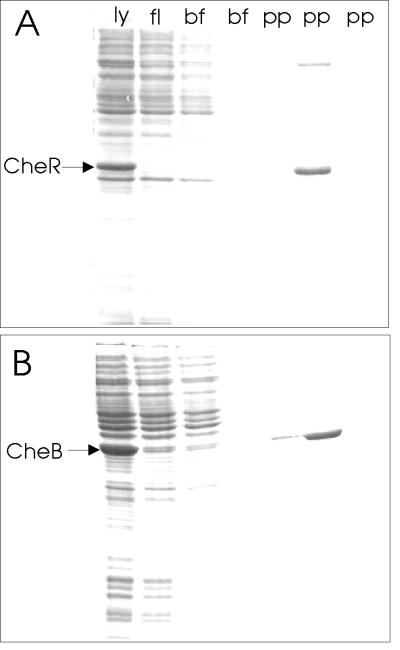
Figure 6.
Retention of modification enzymes by immobilized pentapeptide and elution by free pentapeptide. Soluble lysate (ly) from cells containing CheR (A) or CheB (B) produced from an induced gene located on a multicopy plasmid was applied to a 1-ml column of resin carrying the immobilized pentapeptide, NWETF. Fractions collected during application of the sample (fl), two column volumes of buffer (bf), and three column volumes of buffer containing 5 mg/ml free pentapeptide (pp) were analyzed by SDS/PAGE. The positions of CheR (≈33 kDa) and CheB (≈37 kDa) are indicated on the respective gels. Analysis of the 37-kDa band eluted by pentapeptide in the experiment of B revealed an amino-terminal sequence (seven residues) identical to authentic CheB. Essentially identical results were obtained by using heptapeptides representing the carboxyl-terminal residues of Tsr (EENWETF) or Tar (DPNWETF) as the immobilized or eluting peptide.