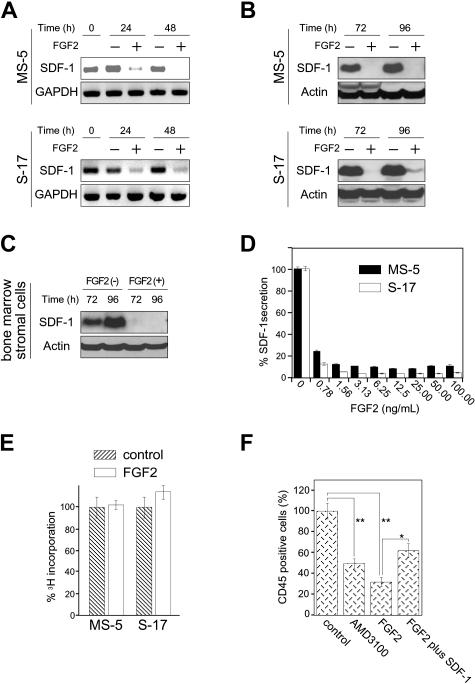
Figure 1.
Effects of FGF2 on stromal cell production of SDF-1. (A) Semiconfluent cultures of MS-5 and S-17 cells were incubated in medium alone or with FGF2 (50 ng/mL). At the indicated time points, total RNA was extracted and tested by semiquantitative RT-PCR for SDF-1 and GAPDH mRNAs (representative experiment of 3). (B) Semiconfluent cultures of MS-5 and S-17 cells were incubated with or without FGF2 (50 ng/mL) for the indicated times. SDF-1 content was measured by Western blotting in cell lysates. After immunoblotting for SDF-1, the membranes were reprobed with antibodies to actin (representative experiment of 3 performed). (C) Semiconfluent cultures of primary bone marrow stromal cells were incubated with or without FGF2 (50 ng/mL) for the indicated times. SDF-1 content was measured by Western blotting in cell lysates. After immunoblotting for SDF-1, the membranes were reprobed with antibodies to actin (representative experiment of 3 performed). (D) MS-5 and S-17 cells were cultured with FGF2 at various concentrations (0-100 ng/mL) for 72 hours; levels of SDF-1 were measured in the culture supernatants. The results reflect the means (± SD) of triplicate determinations (representative experiment of 3 performed). (E) Effects of FGF2 (50 ng/mL) on MS-5 and S-17 cell proliferation. MS-5 cells and S-17 cells were detached, washed twice with PBS, suspended in culture medium (αMEM containing 10% FBS), and incubated (2000 cells/well in 0.2 mL culture medium; triplicate cultures in 96-well plates) with the addition of medium only or medium with FGF2 (50 ng/mL). After 64-hour incubation, DNA synthesis was measured by 3H thymidine deoxyribose uptake. The results, expressed as mean (± SD) cpm/culture, from 3 independent experiments are shown. (F) Effects of exogenous SDF-1 on hematopoietic progenitor cell growth onto stromal cells. MS-5 feeders (12-well plate) were pretreated for 72 hours with or without FGF2 (50 ng/mL). After washing, FGF2-treated feeders were incubated with medium only or with recombinant human SDF-1 (500 ng/mL) for 2 hours. After removal of culture supernatants from all wells, human CD34+ peripheral blood progenitor cells (PBSCs, 1.0 × 105 cells/well, 3 wells/group) were added onto treated and untreated MS-5 cells. CD34+ cells were added in medium alone or medium with AMD3100 (5 μg/mL) onto untreated feeders; and CD34+ cells were added in medium with FGF2 (10 ng/mL) alone or FGF2 plus SDF-1 (500 ng/mL) onto FGF2-treated only and FGF2-treated and SDF-1–replenished feeders, respectively. Cocultures were incubated for 3 weeks with replenishment of culture medium alone (0.5 mL) or with the appropriate additives (10 ng/mL FGF2, 5 μg/mL AMD3100, 10 ng/mL FGF2 plus 500 ng/mL SDF-1) twice/wk. Nonadherent viable cells were counted and analyzed by FACS at the end of incubation. Control indicates untreated MS-5 cells plus untreated CD34+ cells; AMD3100, untreated MS-5 cells plus untreated CD34+ cells plus AMD3100; FGF2, MS-5 feeders treated with FGF2 plus untreated CD34+ cells; and FGF2 plus SDF-1, MS-5 feeders treated with FGF2 plus SDF-1–treated CD34+ cells. The percent growth of PBSCs was calculated as (CD45+ cell number with AMD3100, FGF2, or FGF2 plus SDF-1/CD45+ cell number with no additive) × 100 (%). The results reflect the means (± SD) of 3 independent experiments. The asterisk denotes statistical significance (**P < .01; *P < .05).