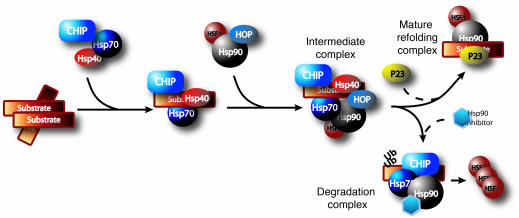
Figure 2. Proposed mechanism of chaperone/client pathway.
A substrate may initially be recognized by the Hsp40/Hsp70 complex with CHIP as the cochaperone/E3 ubiquitin (Ub) ligase. Transfer of the substrate to the Hsp90 complex is facilitated by Hop. There remain 2 fates for the substrate: dephosphorylation and refolding or ubiquitin-dependent proteasomal degradation. The mechanisms dictating which pathway is taken remain undefined. Hsp90 expression inhibits HSF1 activity by direct binding, which prevents HSF1 phosphorylation and trimerization. When Hsp90 is inhibited, Hsp90 levels are decreased, releasing HSF1, which in turn promotes de novo transcription of Hsps.