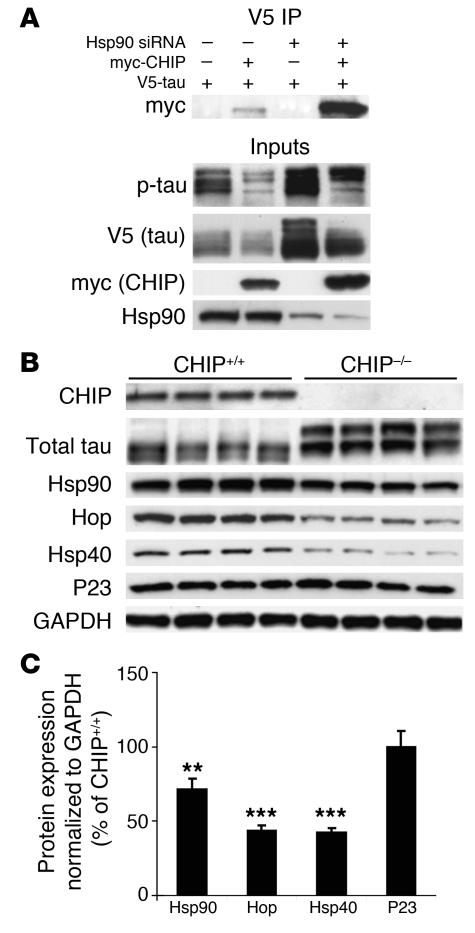
Figure 6. The unique cochaperone CHIP is essential for Hsp90 inhibitor–mediated tau degradation and regulates the levels of other chaperones as well as Hsp90.
(A) HeLa cells transfected with Hsp90 siRNA were subsequently transfected with V5-tau with or without myc-CHIP. Tau accumulated when Hsp90 expression was reduced; however, this accumulation was abrogated by CHIP and the amount of coimmunoprecipitated tau/CHIP complexes increased in the absence of Hsp90. (B) Chaperone protein levels were assessed in CHIP–/– brain tissue by Western blot analysis. The absence of CHIP and elevation in total tau levels were confirmed. Both Hsp40 and Hsp90 levels were decreased. In addition, the non–HSF1-mediated cochaperone, Hop, was also significantly decreased in CHIP–/– mice. P23 levels remained unchanged compared to GAPDH levels. (C) Quantification was assessed by standard densitometry. Error bars represent SD of the 4 CHIP–/– mice. **P < 0.01; ***P < 0.001 versus CHIP+/+.