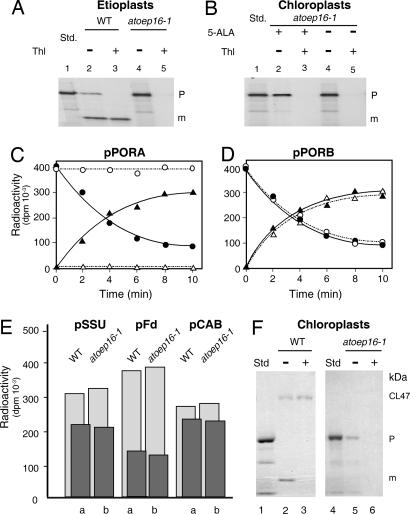
Fig. 4.
In vitro import of 35S precursors into plastids isolated from Atoep16-1 and wild-type seedlings. (A) Import of 35S-pPORA into etioplasts of Atoep16-1 and wild-type plants. The incubations were performed in the presence of 2 mM Mg-ATP. The autoradiograms show precursor (P) and mature (m) protein levels in plastids treated with (+) or without (−) thermolysin (Thl). Std defines input translation standards. (B) As in A, but showing import data of 35S-pPORA for 5-ALA-pretreated, Pchlide-containing, and mock-incubated Atoep16-1 chloroplasts. (C and D) Time courses of import of 35S-pPORA (C) and 35S-pPORB (D) into 5-ALA-treated, Pchlide-containing wild-type (filled circles and filled triangles) and Atoep16-1 (open circles and open triangles) chloroplasts. Precursor and mature proteins are marked by open and filled circles as well as open and filled triangles, respectively. Quantification of radioactivity in protein was done with a PhosphorImager. (E) Quantitative import data for the small subunit precursor of ribulose-1,5-bisphosphate carboxylase/oxygenase (pSSU), precursor ferredoxin (pFd), and the chlorophyll a/b-binding protein precursor of photosystem II (pCAB). Light and dark gray columns refer to precursor and mature protein levels after 15-min import reactions into 5-ALA-treated, Pchlide-containing chloroplasts of wild-type (a) and Atoep16 (b) plants. (F) Cross-linking of 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB)-activated 35S-transA-DHFR, consisting of the Pchlide-responsive transit peptide of pPORA (transA) and a DHFR reporter protein of mouse, to isolated 5-ALA-pretreated, Pchlide-containing wild-type (lanes 1–3) and Atoep16-1 (lanes 4–6) chloroplasts, obtained at 2 mM Mg-ATP and 0.1 mM Mg-GTP. CL47 defines 47-kDa products established between Cys-80 in the DHFR domain of the 31-kDa precursor (P) and Cys-71 in OEP16, and m stands for the imported, mature, 25-kDa DHFR (lane 2). Lane 3 shows a respective immunoprecipitation using OEP16 antiserum. Lanes 5 and 6 depict cross-link products and immunoprecipitates corresponding to those in lanes 2 and 3, respectively, for Atoep16 chloroplasts. In lanes 1 and 4 are shown input translation standards (Std). Note the lack of CL47 and imported, mature DHFR in Atoep16 chloroplasts.