Figure 2.
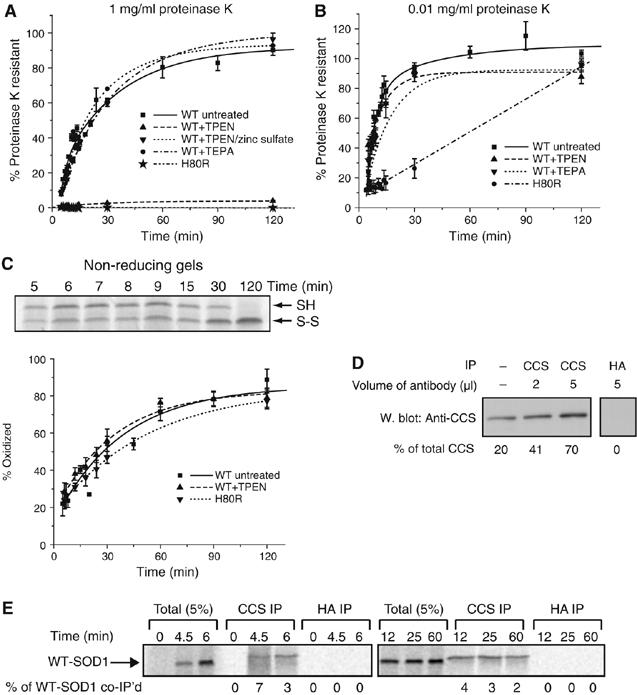
Zinc is required for folding to a compact state but not for disulfide bond formation. Effect of metal chelators on the kinetics of acquisition of resistance of WT-SOD1 to 1 mg/ml (A) or 0.01 mg/ml (B) proteinase K. Samples were prepared and analyzed as in Figure 1B. Proteinase K resistance of zinc-binding mutant H80R is also shown. (C) Top panel, representative non-reducing SDS–PAGE gel of WT-SOD1 showing maturation from mostly reduced to fully oxidized state. Bottom panel, quantification of oxidized fraction of H80R and of WT-SOD1 with or without treatment with 100 μM TPEN, as indicated. Graphical data are mean±s.e.m. from a minimum of three independent experiments per time point. (D) Immunoprecipitation of endogenous CCS from RRL. RRL was immunoprecipitated with human CCS mAb or with control HA mAb, as indicated. Immune complexes were subjected to immunoblotting with a CCS polyclonal antibody and band intensities relative to total CCS were quantified by densitometry, indicated below each lane. Far left lane, loading control of 20% of total RRL in the immunoprecipitations. (E) Co-immunoprecipitation of newly synthesized WT-SOD1 with CCS. Endogenous CCS was immunoprecipitated using the CCS mAb, as in (D), at various times following the translation of newly synthesized WT-SOD1. Anti-HA was used as an immunoprecipitation control. The percentage of newly synthesized WT-SOD1 co-immunoprecipitated with the monoclonal antibodies was quantified via SDS–PAGE/phosphorimage analysis and noted below each lane.
