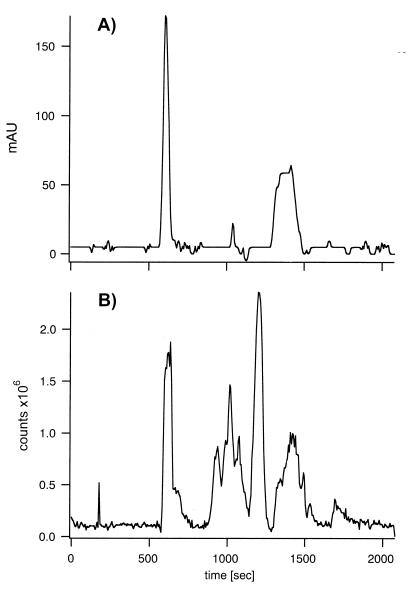
Figure 1.
Chromatographic purification of lactose permease by size-exclusion chromatography in aqueous organic solvent. Purified lactose permease (200 μg protein) was separated from nearly all noncovalently associated lipid and detergent to perform effective ESI-MS. The permease/detergent micelle was disrupted by chloroform/methanol phase separation with the protein precipitating at the interface. Aggregated protein was recovered by centrifugation and dissolved in 90% aqueous formic acid (50 μl), but was still accompanied by residual detergent and lipid. Size-exclusion chromatography in chloroform/methanol/1% aqueous formic acid (4:4:1, vol/vol) was used to separate the permease from these contaminants by using a silica column (G2000SW, 7.8 × 300 mm, TosoHaas) at 40°C and 0.5 ml/min. (A) Elution profile measured at 280 nm (mAU). Permease eluted at 600 sec as a single peak. The eluent is directed to the mass spectrometer, scanning the m/z range 600 to 2,400 every 6 sec. (B) Total ion chromatogram showing the signal detected for each scan versus time. Both chromatograms show separation from residual UV absorbing and ion-generating material.