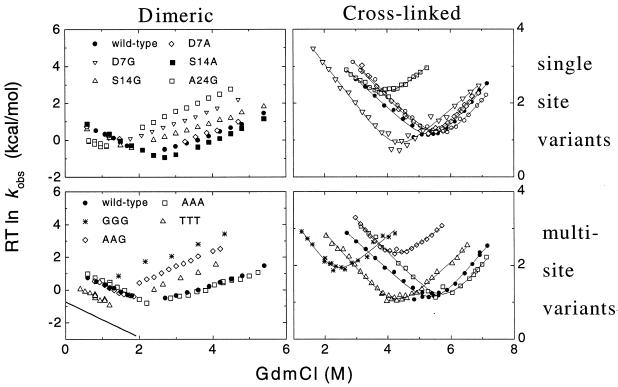
Figure 1.
Chevron plot of folding kinetics of dimeric and crosslinked GCN4-p2′ in 20–100 mM sodium acetate, 150 mM sodium chloride, pH 5.5, 10°C. Symbols are the same in the left and right panels. The measured bimolecular folding rates for the dimeric CC have been scaled to 5.5 μM protein concentration. The solid line in lower left represents the predicted folding rates for the GGG mutant determined from the difference in equilibrium stability and activation energy for unfolding according to ΔG‡f = ΔG‡u + ΔG0. To measure the unfolding rates of the marginally stable dimeric GGG version, 5% (vol/vol) of 2,2,2-trifluoroethanol was added. Previous work demonstrated that 2,2,2-trifluoroethanol does not affect unfolding rates (34).