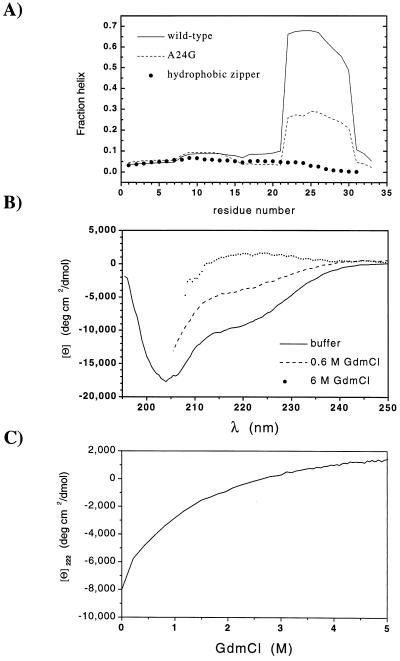
Figure 3.
Residual structure in the denatured state. (A) Predicted helicity for monomers of GCN4-p1′ and the A24G variant at pH 5.5, 10°C, 0.2 M ionic strength, and for the related leucine zipper with a strengthened hydrophobic core (32) at pH 4.8, 25°C, 0.1 M ionic strength, calculated by using agadir (31). (B) CD spectra of an 18-residue peptide encompassing the most helical region of GCN4-p1′ (residues 16–33 with N16D substitution) in 20 mM sodium acetate, pH 5.5, 10°C in 0.2 M NaCl (83 μM); 180 mM NaCl, 0.6 M GdmCl (73 μM), and 6 M GdmCl (43 μM). (C) Denaturation profile of peptide 16–33.