Figure 1. TnT molecules studied.
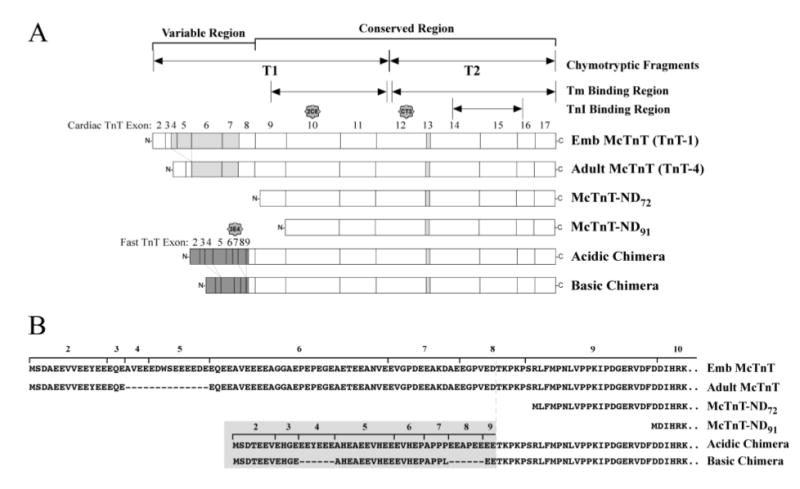
(A) Linear maps show the two naturally occurring mouse cardiac TnT isoforms and the engineered model TnT molecules used in this study. The adult mouse cardiac TnT (TnT-4) differs from the embryonic cardiac TnT (TnT-1) by the exclusion of exons 4 and 5 from the NH2-terminal variable region. The truncated mouse cardiac TnT molecules deleted only the NH2-terminal variable region encoded by exons 2-8 (McTnT-ND72) or further including a conserved segment encoded by exon 9 (McTnT-ND91). The two chimeric TnT molecules are identical to the mouse cardiac TnT in their central and COOH-terminal regions (exons 9-17) but contain the NH2-terminal domain of an acidic chicken fast skeletal TnT (encoded by exons 2-8 from chicken fast skeletal TnT-3) (Acidic chimera) or a basic chicken fast skeletal TnT (encoded by exons 2-3, 5-7 from chicken fast skeletal TnT-4) (Basic chimera). The TnI binding region, tropomyosin binding regions, T1 and T2 chymotryptic fragments and the mAb 3E4, CT3 and 2C8 epitopes are designated. Light grey exons indicate alternately spliced exons in cardiac TnT; dark grey exons indicate chicken fast skeletal muscle TnT exons. (B) The NH2-terminal amino acid sequences of the six TnT molecules are aligned to demonstrate the structural relationships. Residues originated from chicken fast skeletal TnT are highlighted in grey.
