Figure 2. TnT protein preparations.
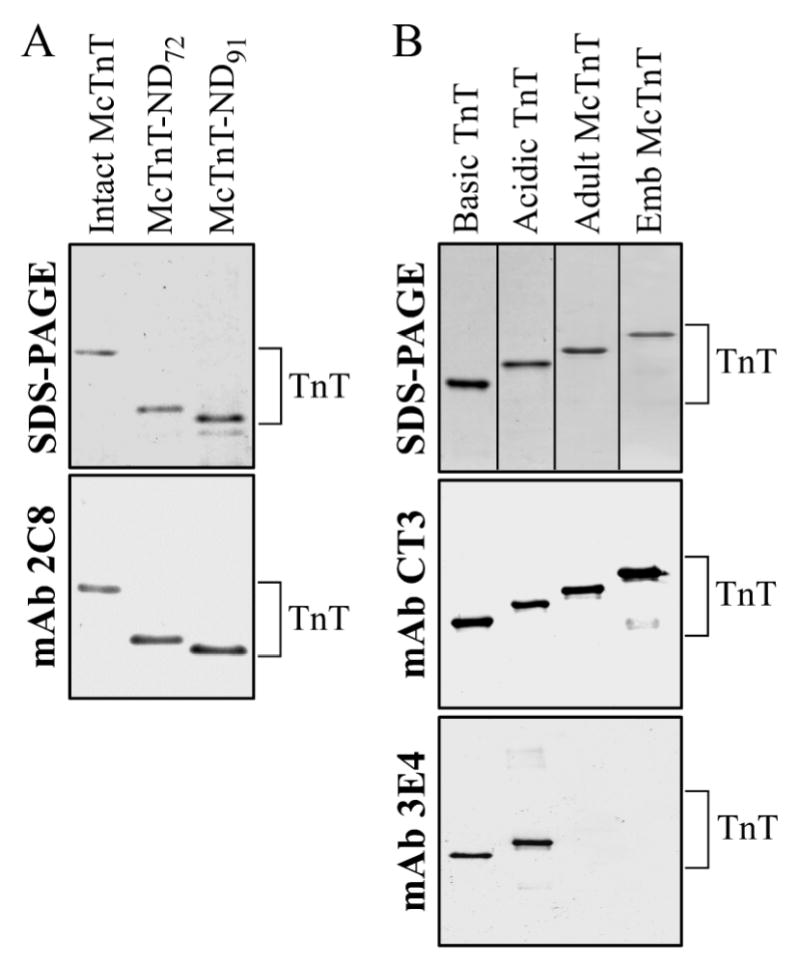
Recombinant TnT proteins were expressed in E. coli and purified as shown by the SDS-PAGE gels. (A) NH2-terminal truncated TnT (McTnT-ND72 and McTnT-ND91) were analyzed along with the intact adult mouse cardiac TnT-4 (Intact McTnT). The Western blot demonstrates that the truncated TnT proteins are positive to mAb 2C8 that recognizes an epitope in the exon 10 region (Fig. 1). (B) The Basic and Acidic chimeric TnTs were analyzed along with the adult mouse cardiac TnT-4 (Adult McTnT) and embryonic mouse cardiac TnT-1 (Emb McTnT). The Western blot demonstrates that the chimeric TnTs same as the native cardiac TnTs are positive to mAb CT3 recognizing a central cardiac TnT epitope. The chimeric TnTs are also positive to mAb 3E4 that recognizes an epitope within exon 7 of chicken fast skeletal muscle TnT. The results verified the construction of the NH2-terminal deleted and recombinant chimeric TnT molecules.
