Figure 3. Selective deletion of the NH2-terminal variable region from TnT does not abolish binding to Tm.
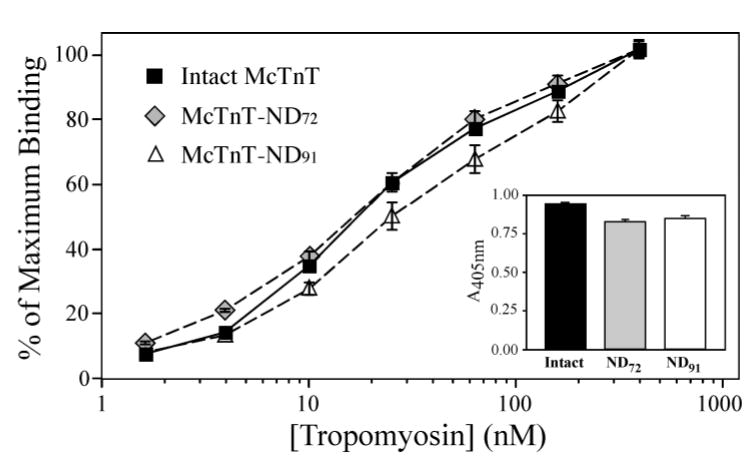
Solid-phase protein binding curves of intact mouse cardiac TnT, McTnT-ND72 and McTnT-ND91 to Tm demonstrate the effect of TnT NH2-terminal truncations. The binding affinity of McTnT-ND72 to Tm was similar to that of the intact McTnT while the Tm binding affinity of McTnT-ND91 was decreased (P<0.001). There was no significant change in the maximal binding of McTnT-ND72 and McTnT-ND91 to Tm (inset). In contrast to the preserved activity of McTnT-ND72 with only the NH2-terminal variable region deleted, the decreased binding of McTnT-ND91 to Tm indicates that deletion into the conserved region damages the TnT core structure by disrupting the central Tm binding site (Fig. 1).
