Figure 7. Alternative splicing-generated NH2-terminal charge difference in cardiac TnT affects binding affinity to Tm and TnI.
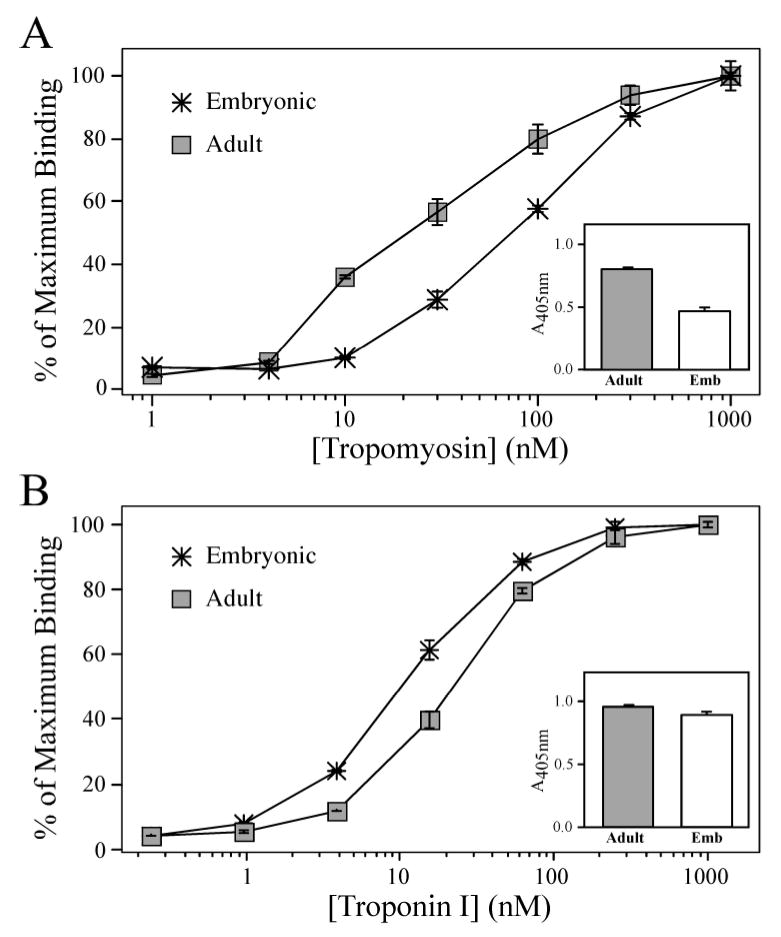
(A) Solid-phase protein binding curves demonstrate that the less acidic Adult mouse cardiac TnT immobilized to microtiter plate bound with a higher affinity to Tm than that of the more acidic Embryonic cardiac TnT, as determined by the concentration of Tm required for 50% maximum binding (P < 0.05). The Adult cardiac TnT also exhibited a higher level of maximal binding to Tm than that of the embryonic cardiac TnT (shown by the A405nm values in the inset). (B) However, the Adult cardiac TnT bound to TnI with a lower affinity compared to that of the Embryonic cardiac TnT (P < 0.05), while the A405nm values in the inset show the Adult cardiac TnT demonstrated a moderate but statistically significant higher maximal binding to TnI compared to that of the Embryonic cardiac TnT (P < 0.05).
