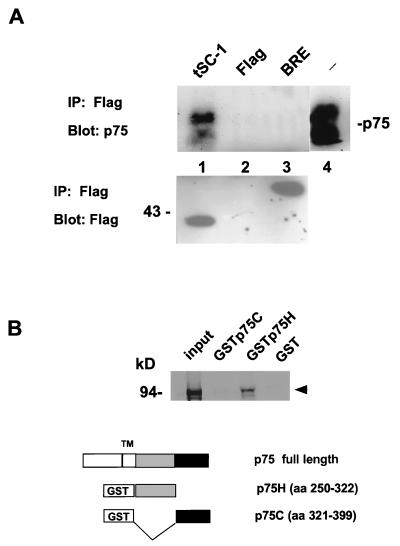
Figure 2.
Interaction of p75 and SC-1. (A) Transfected 293 cells expressing the rat p75 cDNA; an unrelated protein, Flag-tagged BRE, and a Flag-tagged truncated SC-1 cDNA (pSC2; amino acids 490–814; Fig. 1B) were subjected to immunoprecipitation using anti-Flag antibodies, followed by blotting with the anti-p75 antibody, 9992. The arrowheads demonstrate the position of p75 on the blot, which migrated as a doublet, representing products of differential glycosylation of p75. Lanes: 1, p75 and pSC2; 2, p75 and Flag vector; 3, p75 and an unrelated protein Flag-Bre cDNA (22); 4, 293 cells transfected with p75 cDNA alone, probed with anti-p75, 9992 antibody. In the lower panel, the transfected proteins were detected after immunoprecipitation and Western blotting with anti-Flag antibodies. (B) SC-1 interacts with the juxtamembrane domain of human p75. GSTp75 fusion proteins were used for assessing the binding of in vitro translated SC-1 to p75 intracellular sequences. The binding reaction was carried out by using 3 μg of the GSTp75 fusion proteins on glutathione beads. The left-most lane represents input in vitro translated SC-1 protein. The proteins were resolved on a 7% SDS/PAGE gel. The arrowhead indicates the migration of the major in vitro translated product of SC-1.