Figure 1.
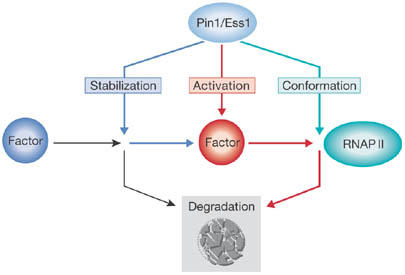
The peptidyl-prolyl isomerase Pin1/Ess1 acts in three ways in transcriptional activation. In the first scenario (blue), PPI activity protects client proteins such as β-catenin and p53 from proteasomal degradation, allowing them to participate in promoter binding and gene activation. In the second scenario (red), conformational change triggers ubiquitin-dependent degradation by the proteasome (grey box), which might (for example, in the case of retinoic-acid receptor α or steroid-receptor coactivator-3) or might not (for example, in the case of c-Myc or interferon-regulatory factor 3) be linked to transcriptional potency. The third scenario (turquoise) involves direct interaction of Pin1 with the carboxy-terminal domain of RNA polymerase II (RNAP II), which has been implicated in downregulation of the polymerase during mitosis, polymerase recycling and elongation. PPI, peptidyl-prolyl isomerase.
