Figure 1.
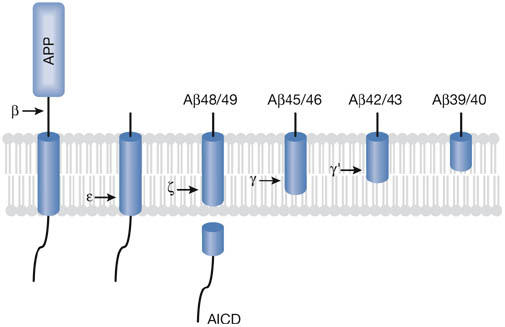
Ihara model of processive proteolysis of the amyloid precursor protein transmembrane domain by γ-secretase, beginning at the ε-cleavage site and cleaving every three residues. This model explains how reduction of proteolytic function owing to presenilin mutations might lower amyloid β-peptide (Aβ) production but increase the ratio of Aβ42 to Aβ40. Longer forms of Aβ, with more of the hydrophobic transmembrane domain, might be more likely to be retained in the active site of the protease, whereas the shorter forms are more likely to be released. Less catalytically efficient γ-secretase complexes would allow more time for the release of longer Aβ peptides. In addition, Alzheimer disease-causing presenilin mutations shift the initial ε-cleavage site to produce more Aβ48, which would lead to Aβ42. AICD, APP intracellular domain; APP, amyloid precursor protein.
