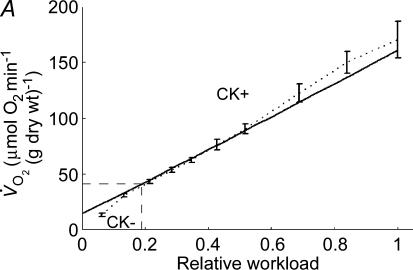
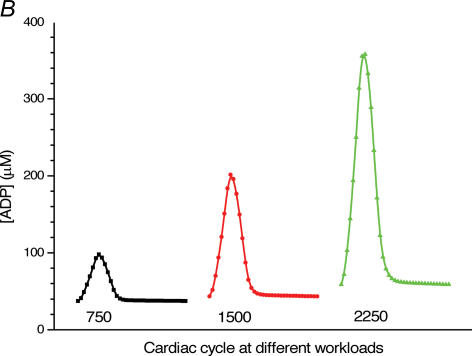
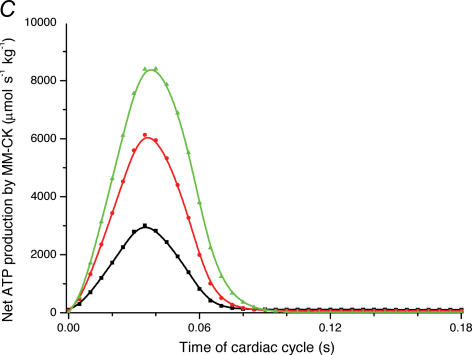
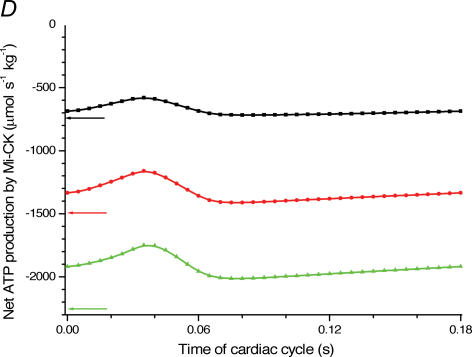
Figure 4. Mathematical modelling of the regulation of mitochondrial respiration in cardiac cells in vivo under physiological conditions controlled by the Frank-Starling mechanism.
A, computed (continuous line) and experimental (points with standard deviations, from Williamson et al. (1976) oxygen consumption rates of working cardiac muscle. Relative workload is the fraction of maximal workload applied, in computations this is the rate of ATP hydrolysis by actomyosin–MgATPase. CK+ shows the calculations for active creatine kinase system, CK- shows the calculations for the cells with inactivated creatine kinases (results shown within the dashed square). B, ADP profiles in the myoplasmic space in the core of myofibrils of ICEUs over cardiac cycle at different workloads (in the presence of CK). The model includes the diffusion restriction of ADP through mitochondrial outer membrane (Vendelin et al. 2000). Profiles correspond to the workloads equivalent to 750 (black), 1500 (red) and 2250 (green) μmol ATP s−1 kg−1. Reproduced from Vendelin et al. (2000) with permission. C, workload dependence of the dynamics of net ATP production by MM-CK in myofibrils. The reaction rates for workloads of 750 (black), 1500 (red) and 2250 (green) μmol ATP s−1 kg−1 are shown. Net rate of the reaction means displacement from the equilibrium position of the creatine kinase reaction (observed in diastolic phase) as the result of local ADP production in the contraction cycle (Fig. 4B). Courtesy of M. Aliev. D, workload dependence of the dynamics of net PCr production by mito-CK. Workload values are indicated by the arrows of respective colour. Complete model of Dos Santos et al. (2000), used in simulations in Saks et al. (2005). Mitochondrial membrane potential ΔΨ=−160 mV, Vmax of ATP-synthase = 4269.3 μmol ATP s−1 kg−1. PCr export by mitochondria is 90.9, 89.1 and 85.8% of total mitochondrial energy export for workloads of 750, 1500 and 2250 μmol ATP s−1 kg−1, respectively. Due to the functional coupling between ANT and MtCK (Fig. 2), the reaction runs always out of equilibrium in the direction of PCr synthesis (Vendelin et al. 2004b), the steady-state values of the rates of this coupled reactions are increased by increasing the workload (cyclic changes of MgATP and MMCK reactions in myofibrils) and metabolic signalling of these changes to mitochondria by the creatine kinase–phosphocreatine energy transfer system (see Fig. 2 and the text). Courtesy of M. Aliev.