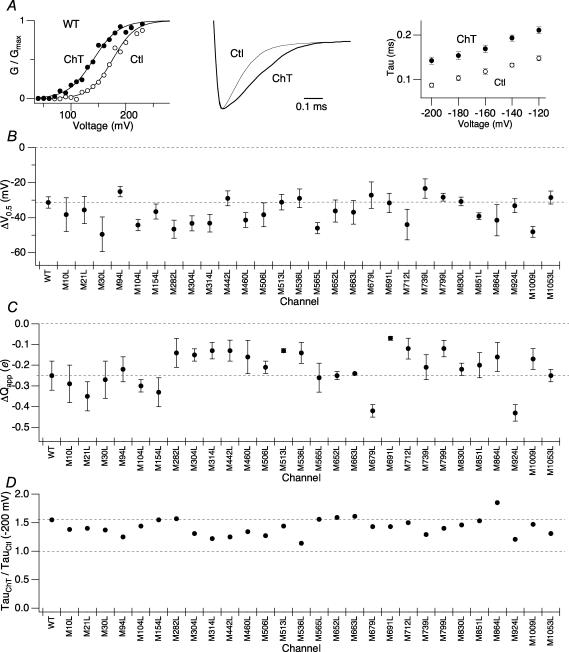
Figure 2. Individual mutation of single methionine residues within hSlo1 does not remove the functional sensitivity to oxidation by Ch-T.
A, left: representative wild-type hSlo1 G–V curve before (○) and after (•) modification by 2 mm Ch-T. The macroscopic currents were elicited by pulses to different test voltages from the holding voltage of 0 mV. Centre: scaled tail currents recorded at −200 mV after pulses to 180 mV before (thin sweep) and after (thick sweep) Ch-T modification. Right: voltage dependence of the deactivation time constants for wild-type hSlo1 before (○) and after (•) application of Ch-T. B, mean ΔV0.5 and C, ΔQapp caused by treatment with Ch-T from the single point-mutant channels compared with those of wild-type hSlo1. See Table 1 for mean ±s.e.m. values. See Supplementary Fig. 1A for single point-mutant G–V curves and results regarding the M285L mutant. D, relative increase in the time constant due to treatment with Ch-T measured at −200 mV for the single point-mutant channels compared with that of wild-type hSlo1. See Table 2 for mean ±s.e.m. values; n = 3. See Supplementary Fig. 1B for single point-mutant scaled tail currents and Supplementary Fig. 1C for the voltage dependence of the deactivation time constants of the single point-mutant channels.