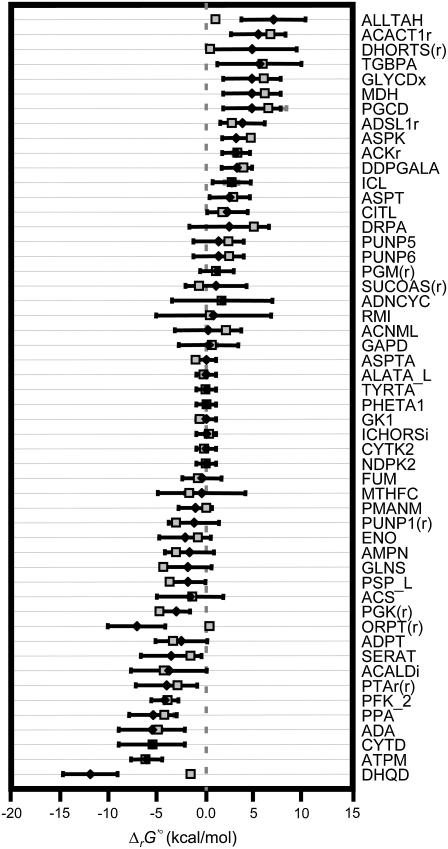
FIGURE 1.
Comparison of estimated ΔrG′° to experimentally measured ΔrG′°. Estimated ΔrG′° values (solid diamonds) are shown and compared to experimentally measured ΔrG′° values (shaded squares) for every reaction in the iJR904 model for which data exists in the NIST database (39) within the temperature and pH limitations (288–308 K and pH of 6–8). In some cases, multiple data points existed in the NIST database at the suitable conditions. In these cases, the average ΔrG′° is shown along with the standard deviation in the data (shaded error bars). The uncertainty in the estimated ΔrG′° (solid error bars) calculated from the group contribution method (22) is also shown verifying that all but three of the measured ΔrG′° fall within the range of uncertainty of the estimated ΔrG′°.