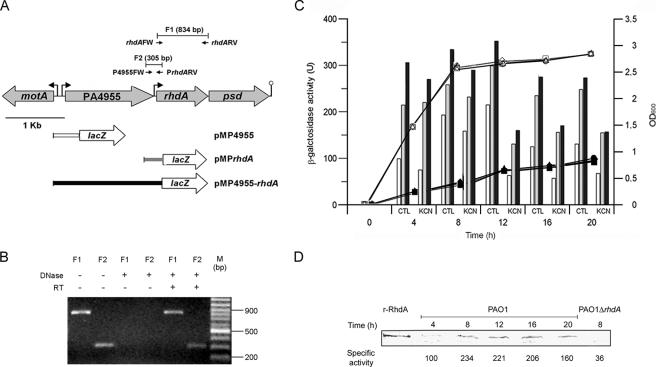
FIG. 3.
Identification of rhdA promoter regions and regulation of rhdA expression. (A) Schematic representation of the rhdA locus. Gray arrows show the orientations of ORFs. Small black arrows show the positions and orientations of RT-PCR primers. The lengths of the predicted RT-PCR products (F1 and F2) are reported. Bent arrows and the lollipop show the locations of putative promoters and the transcription terminator, respectively. Gene fusions with lacZ are shown in the lower part (see Materials and Methods and Table 1). For clarity, inserts of pMP4955 (white), pMPrhdA (gray), and pMP4955-rhdA (black) have the same color as the corresponding bars in the histograms in panel C. (B) Detection of rhdA and PA4955-rhdA transcripts by RT-PCR. Lanes show RT-PCR products amplified from total RNA isolated from P. aeruginosa PAO1 grown in LB for 12 h. PCRs were with or without DNase or reverse transcriptase pretreatment as indicated; F1 and F2 indicate the predicted RT-PCR products (see panel A). M, molecular size standard. (C) Activity of rhdA promoter regions and regulation of rhdA expression. Bacterial growth (OD600 on the right ordinate) was determined at 37°C in LB medium with (black symbols) or without (white symbols) 300 μM KCN. Circles, PAO1(pMP4955); triangles, PAO1(pMPrhdA); squares, PAO1(pMP4955-rhdA). The histogram shows the LacZ activity (on the left ordinate) of pMP4955 (white), pMPrhdA (gray), and pMP4955-rhdA (black) transcriptional fusions in P. aeruginosa PAO1 grown in LB medium at 37°C with (KCN) or without (control [CTL]) 300 μM KCN. The background LacZ activity for PAO1(pMP220) was ca. 20 U. Values are the averages from three independent assays. Standard deviations are <9% of the given values. (D) Time course analysis of RhdA expression by P. aeruginosa PAO1 as revealed by immunoblot analysis of RhdA levels in the cytoplasmic fraction (35 μg of total protein) of bacteria grown in LB at 37°C. The specific rhodanese activity (nmol min−1 mg−1 of cytoplasmic protein) at each time point is also reported. Purified recombinant RhdA (r-RhdA) (150 ng) and cytoplasmic proteins (35 μg) from P. aeruginosa PAO1 ΔrhdA were used as the positive and negative controls, respectively. Blots were hybridized with a mouse anti-RhdA polyclonal antiserum.