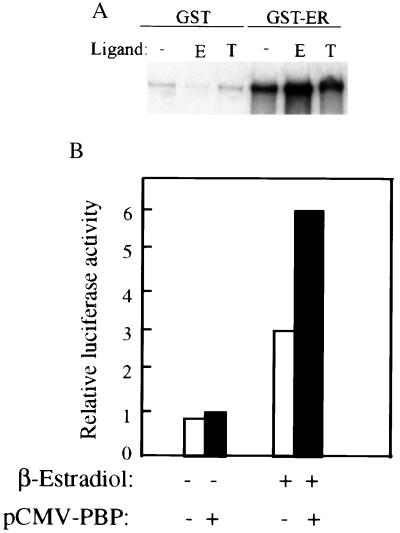
Figure 1.
Binding of PBP to ER and potentiation of estrogen dependent transcription from ER. (A) Binding of PBP to ER. [35S] methionine-labeled PBP generated by in vitro translation was incubated with glutathione-Sepharose beads containing GST, or GST-ER, in the presence of estrogen (E) or tamoxifen (T) or absence of the ligand (-). The bound protein was eluted and analyzed by SDS/PAGE and was autoradiographed. PBP binds to ER in the absence of estrogen and in the presence of tamoxifen, but estrogen increases the interaction of ER with PBP. No binding was seen for GST alone, indicating that the interaction is specific for ER. (B) PBP potentiates the transcriptional activity of ER. CV-1 cell were transiently cotransfected with 1.5 μg of reporter construct ERE-TK-luc, 0.25 μg of pCMV-ER, 0.5 μg of pCMX-PBP or pCMX, and 0.5 μg of pCMVβ in the presence of β-estradiol or absence of ligand. PBP increased the transcription of luciferase gene ≈2- fold in the presence of β-estradiol. Results are the mean of four independent transfections and are normalized to the internal control β-galactosidase expression.