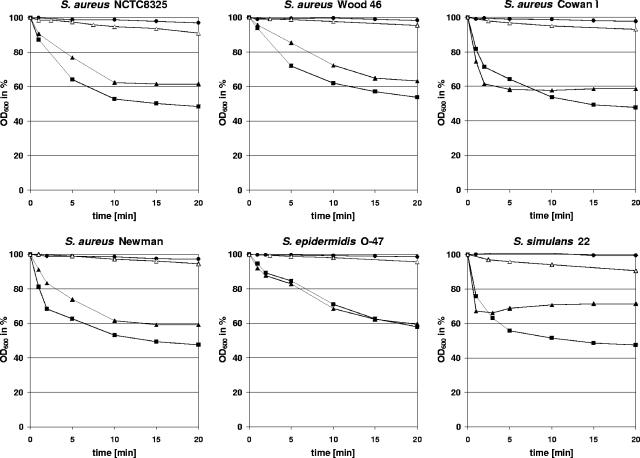
FIG. 4.
The φ11 endolysin lyses whole cells of several staphylococci. Light scattering data showed that the φ11 endolysin (▴; 20 μg/ml) rapidly decreased the optical density of heat-inactivated S. aureus NCTC8325, S. aureus Wood 46, S. aureus Cowan 1, S. aureus Newman, S. epidermidis O-47, and S. simulans 22 cultures compared to the protein eluate of the empty-vector control (•). Its lytic activity was comparable to the effect observed after addition of lysostaphin (▪; 5 μg/ml), which is a highly efficient glycyl-glycine endopeptidase. After deletion of the cell wall targeting domain, the φ11 enzyme (φ11endo/ami, ▵; 20 μg/ml) showed a significantly reduced activity, which indicates a fundamental role of the cell wall binding domain in efficient cell lysis.