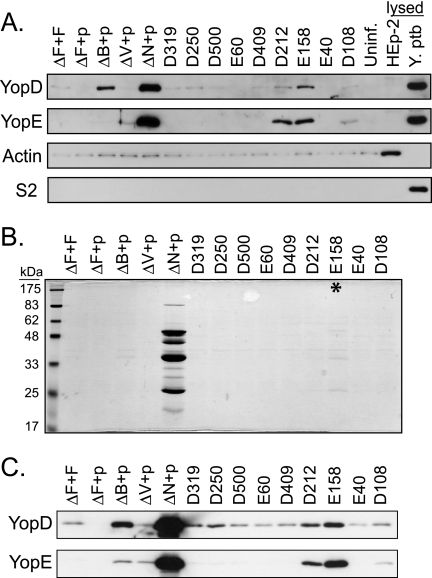
FIG. 5.
Most yscF TD mutants maintain tight needle-translocon connections during infection. Yersinia strains containing pTRC99A or pTRC99A expressing various yscF TD mutants were grown in 2× YT media with 5 mM calcium for 2 h at 26°C, induced with IPTG, and shifted to 37°C for 2 h. (A) Bacteria were added to monolayers of HEp-2 cells at an MOI of 50:1 and centrifuged to initiate cell contact and subsequent Yops translocation. After 1 hour of infection, tissue culture supernatants were collected and centrifuged to remove bacteria. Proteins were precipitated with TCA, separated by SDS-PAGE, and analyzed by Western blotting with antisera to the YopE and YopD proteins. Controls for disrupted HEp-2 cells and bacteria included uninfected cells lysed with Triton X-100 (lysed HEp-2) and bacteria solubilized in SDS-containing sample buffer (lysed Y. pseudotuberculosis [Y. ptb]). Antisera to actin were used to detect leakage of HEp-2 cytosol during infection, and antisera to the ribosomal subunit S2 were used to detect lysis of bacteria. (B) Secreted proteins in the presence of calcium. Y. pseudotuberculosis bacteria were centrifuged, and culture supernatants were collected. Secreted proteins were precipitated with TCA, separated by SDS-PAGE, and stained with Coomassie blue. Molecular mass standards are shown on the left. The asterisk points to the E158 mutant with barely detectable secreted Yops. (C) Western blot of secreted proteins from panel B, probed with antiserum to YopE and YopD.