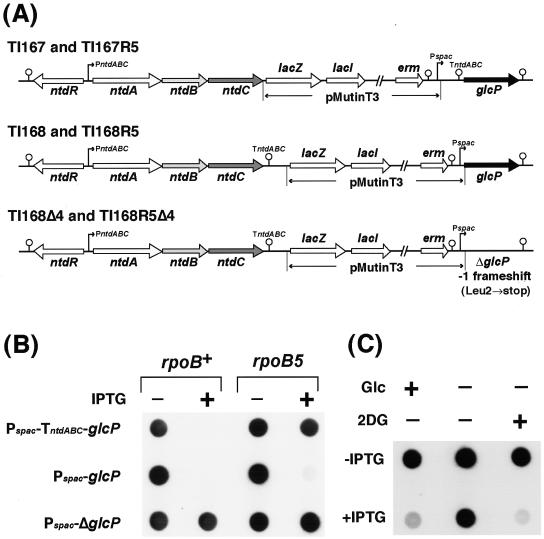
FIG. 5.
Repression of ntdABC transcription by GlcP-mediated glucose transport. (A) Construction of glcP-interrupted strains. B. subtilis glcP-interrupted strains carrying ntdC::pMutinT3 or glcP::pMutinT3 were constructed by integrating a plasmid, pMutinT3, immediately upstream or downstream of TntdABC, respectively. These strains enabled us to control the expression of the glcP gene via the IPTG-dependent Pspac. A glcP-disrupted strain carrying ΔglcP::pMutinT3 also was constructed. In this strain, T nucleotide in the second codon of glcP was deleted, resulting in a −1 frameshift and a nonsense mutation at Leu-2 (TTA) (→ochre [TAA]). (B) Repression of ntdABC by induction of glcP. Total cellular RNAs were prepared from cells of strains TI167 (rpoB+ ntdC::pMutinT3), TI168 (rpoB+ glcP::pMutinT3), TI168Δ4 (rpoB+ ΔglcP::pMutinT3), TI167R5 (rpoB5 ntdC::pMutinT3), TI168R5 (rpoB5 glcP::pMutinT3), and TI168R5Δ4 (rpoB5 ΔglcP::pMutinT3), which were grown for 10 h in S7N medium containing excess (1%) glucose with or without 2 mM IPTG. RNA samples (10 μg each) were spotted directly onto a membrane and hybridized with the RNA probe for ntdB. (C) Repression of ntdABC by GlcP-mediated sugar transport. B. subtilis strain TI168 (rpoB+ glcP::pMutinT3) was grown in S7N medium containing excess (1%) glucose (Glc) until OD650 reached 1.0, after which the cells were harvested by centrifugation and transferred into the same S7N medium (left lane), S7N medium without glucose (center lane), or S7N medium supplemented with 1% 2-deoxy-glucose (2DG) instead of glucose (right lane). Where indicated, IPTG was added to final a concentration of 2 mM just after the cells were transferred. After incubation for an additional 4 h, cells were harvested and RNA samples (4 μg each) were spotted directly onto a membrane and hybridized with the RNA probe for ntdC.