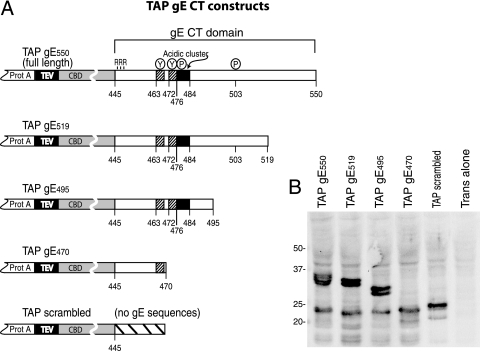
FIG. 4.
Construction of TAP-gE fusion proteins. (A) The TAP domain was composed of two tandem protein A (IgG-binding) domains separated from a CBD by a tobacco etch virus protease cleavage site. The TAP domain was fused N-terminal of the entire gE CT domain, beginning with three arginine residues that are adjacent to the gE transmembrane domain. Other TAP fusion proteins included truncated versions of the gE CT domain: gE519, gE495, and gE470. Also shown is a construct designated TAP scrambled, which contains 25 random amino acids unrelated to the gE CT domain. (B) Vero cells were coinfected with Ad vectors expressing TAP/gE550, TAP/gE519, TAP/gE495, TAP/gE470, or TAP scrambled and Adtet-trans or with Adtet-trans alone. Cell extracts were subjected to electrophoresis on polyacrylamide gels, and then proteins were transferred to the PVDF membrane and probed with an anti-CBD rabbit antibody. Molecular mass markers of 50, 37, 25, and 20 kDa are indicated.