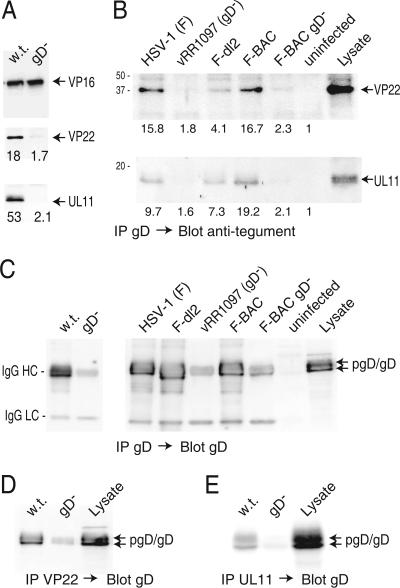
FIG. 8.
Coimmunoprecipitation of VP22 and UL11 with gD from HSV-infected cells. (A) HaCaT cells were infected with F-BAC (w.t.) or F-BAC gD− for 12 h, and then cell extracts were made using 0.5% NP-40 lysis buffer. gD was immunoprecipitated using MAb DL6, proteins were subjected to electrophoresis and then transferred to membranes, and the membranes were probed with rabbit polyclonal anti-VP16 (upper panel), anti-VP22 (middle panel), or anti-UL11 (lower panel) antibodies. (B) HaCaT cells were infected with wild-type HSV-1 strain F, vRR1097 (a gD-null mutant), F-dl2 (expressing a gD lacking the CT domain), F-BAC, or F-BAC gD− for 12 h or were left uninfected. Cell extracts were made using 0.5% NP-40 lysis buffer, and gD was immunoprecipitated using anti-gD MAb DL6. Precipitated proteins and a sample representing 20% of the cell extract (Lysate) were subjected to electrophoresis, transferred to membranes, and probed with anti-VP22 or anti-UL11 rabbit antibodies. The numbers shown in panels A and B were derived as described in the legend to Fig. 7, with the background value (set at 1) corresponding to the blot regions with no obvious proteins. (C) Samples immunoprecipitated as described for panels A and B were also probed with rabbit anti-gD antibodies. The IgG-heavy and -light chains (indicated by HC and LC, respectively) derived from mouse MAb DL6 used to precipitate gD were detected through cross-reaction with secondary antibodies and were most obvious in samples from cells lacking gD. (D and E) VP22 or UL11 was precipitated using rabbit polyclonal antibodies, and samples were blotted with mouse anti-gD MAb DL6. In panel D, some IgG-heavy chains were detected by the secondary antibodies, most obviously in samples from the gD− mutant.