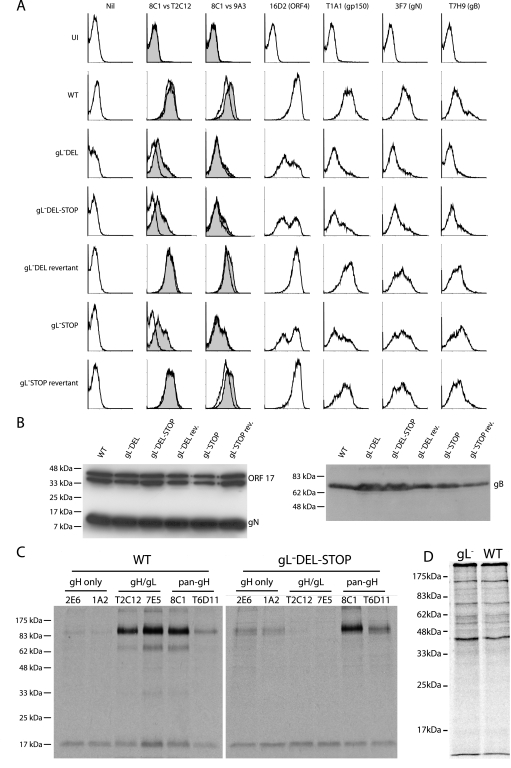
FIG. 3.
Glycoprotein expression by gL-deficient MHV-68. A. BHK-21 cells were left uninfected (UI) or infected (1 PFU, 24 to 48 h) with gL− or gL+ viruses as indicated. The cells were then trypsinized and stained for viral glycoproteins. nil, secondary antibody only. For gH expression, gH/gL-specific (T2C12) and gH-only (9A3) MAbs (open histograms) are shown in comparison to a pan-gH MAb (8C1) (filled histograms). eGFP-expressing viruses were used, and each histogram shows an equivalent eGFP+-gated population to ensure equivalent levels of infection. WT, wild type. B. Viral proteins (equivalent to 5 × 104 PFU/lane for each virus) were denatured, resolved by SDS-PAGE, and immunoblotted for gB and gN as indicated. MAb 7D1-C12 specifically detects ORF17, either in cells transfected with an ORF17 expression plasmid or as a glutathione S-transferase-ORF17 fusion protein expressed in Escherichia coli (data not shown). It provides a loading control. The doublet band reflects the fact that ORF17 is autoproteolytically cleaved. C. gH was immunoprecipitated from 35S-labeled virions. The MAbs used were gH only (2E6, 1A2), gH/gL specific (T2C12, 7E5), or pan-gH (8C1, T6D11). gH migrates at an apparent molecular mass of 85 kDa. The nonspecifically immunoprecipitated 19-kDa band probably corresponds to the abundant ORF65 capsid component. D. Input labeled virion proteins used for immunoprecipitation in panel C.