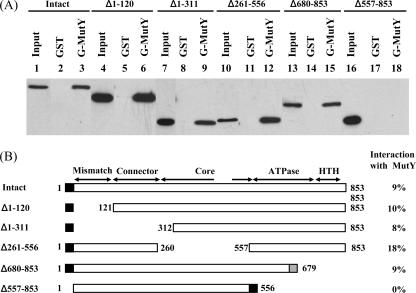
FIG. 4.
Determination of regions within MutS involved in MutY binding. (A) The GST pull-down assays were performed with different MutS constructs as indicated and GST-MutY immobilized on glutathione-Sepharose (lanes 3, 6, 9, 12, 15, and 18). Controls were run concurrently with immobilized GST alone for each MutS construct (lanes 2, 5, 8, 11, 14, and 17). Ten percent of each input MutS construct was loaded to lanes 1, 4, 7, 10, 13, and 16. Western blot analyses of the pellets were performed with antibody to His tag. (B) Graphic depiction of MutS constructs and the binding to GST-MutY fusion protein. The amino-terminal mismatch recognition domain (residues 2 to 115), the connector domain (residues 116 to 266), the core domain (residues 267 to 443 and 504 to 567), the ATPase domain (residues 568 to 765), and the HTH domain (residues 766 to 800) are indicated. Residues 444 to 503 are the clamp domain. The names of MutS domains with their corresponding residues were adapted from the work of Lamers et al. (30). In addition, the extreme C-terminal domain (residues 801 to 853) is responsible for the tetramer formation (5). The portions of protein present in the MutS deletion constructs are indicted by boxes and numbers of amino acid residues. The black and gray boxes at the N or C terminus of MutS represent His tag and an additional 13 amino acids, respectively. The strength of binding, presented at the right, was calculated as the ratio of the amount of MutS in the pellet to that of input MutS constructs.