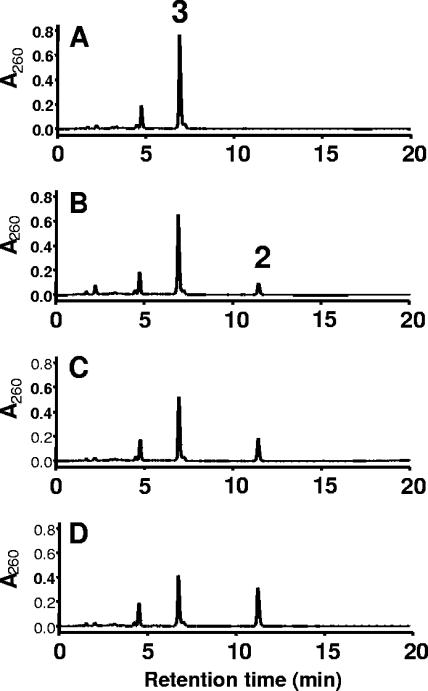
FIG. 3.
Conversion of 6-OH-cyclohexa-1-ene-1-carbonyl-CoA and cyclohex-1-ene-1-carbonyl-CoA (0.5 mM each) by purified BamRGeo. The numbers of the peaks refer to the structures shown in Fig. 1. Shown is the time-dependent dehydration of 6-OH-cyclohexenoyl-CoA (peak 3) to dienoyl-CoA (peak 2) from HPLC analysis of samples taken at 0 s (A), 15 s (B), 45 s (C), and 180 s (D). The reaction leveled off after 3 min by reaching the 1:1 equilibrium between substrate and product. Detection of all compounds was carried out by a UV monitor at 260 nm.