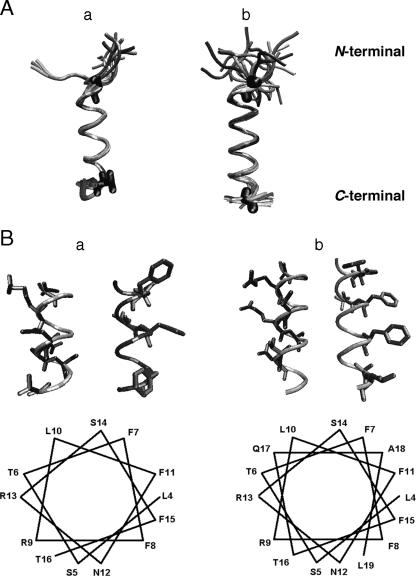
FIG. 2.
(A) Retained structures of TPC3 (a) and UA159sp (b) determined from NMR analysis and computer simulations. The structures that did not violate any structural constraints were retained. The N termini of both peptides are in randomly coiled conformations, but the core helical regions are comparable to each other (residues 4 and 20 for UA159sp and residues 5 and 16 for TPC3). (B) α-Helical wheel of TPC3 (a) and UA159sp (b). The hydrophobic residues are clustered around two-fifths of the α-helix, whereas the basic and hydrophilic residues comprise the remaining three-fifths. Only the α-helical portion of the peptides is shown. Note that TPC3 lacks the proposed C-terminal motif but still has the amphipathic α-helical structure.