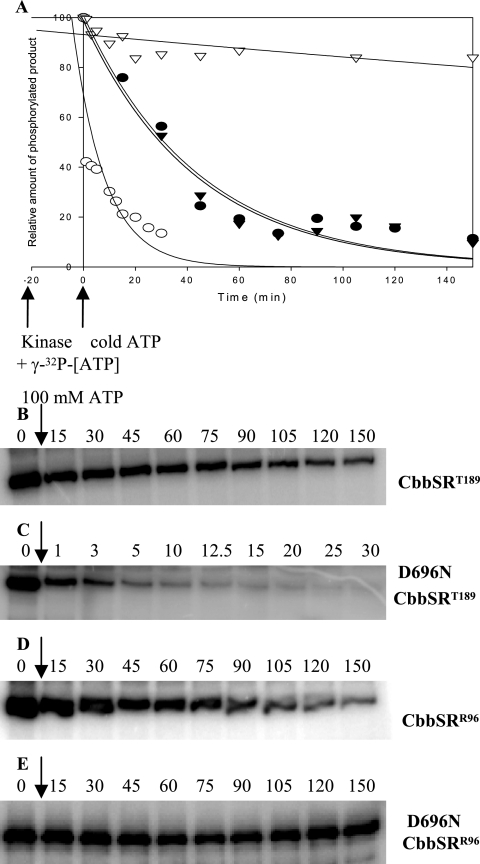
FIG. 7.
Pulse-chase analysis of the CbbSRR96 and CbbSRT189 wild-type truncated histidine kinase and their D696N site-directed mutants. The phosphorylation levels of the four constructs were normalized to the maximum intensity of signal (100%) of each construct prior to the addition of an excess of unlabeled (100 mM) ATP and plotted against time. Label disappearance (γ-32P) is expressed as the percentage of the phosphorylated products obtained at any given time after the addition of unlabeled ATP. The kinase proteins were preincubated with 20 μM [γ-32P]ATP for 20 min, with the exception of D696N CbbSRR96, which was preincubated with 25 nM [γ-32P]ATP. The reaction was carried out in a 100-μl final volume. At time zero, 10 μl of the phosphorylation reaction mixture was removed, and 10 μl of unlabeled ATP (100 mM final concentration) was added. Aliquots were removed at the time points indicated. CbbSRT189, (•); D696NCbbSRT189, (○); CbbSRR96, (▾); D696NCbbSRR96, (▿). Raw data were analyzed, normalized, and plotted with SigmaPlot 8.0. Representative pulse-chase patterns of phosphorylation are shown for CbbSRT189 (B), D696NCbbSRT189 (C), CbbSRR96 (D), and D696NCbbSRR96 (E).